Common characteristics of aldehydes and ketones
Aldehydes and ketones have characteristic reactions in common due to the presence of the carbonyl group in both, but they also have distinct reactions due to the property of aldehydes to be oxidized to acids by the action of mild oxidants. The analysis of aldehydes and ketones focuses on the presence of the carbonyl group is detected by the appearance of an absorption band in the IR spectrum (which is usually the most intense of the spectrum) in the region of 1650 to 1850 cm-1. They differ from esters by the absence of two intense bands in the 1050 to 1250 cm-1 region that appear in esters.
Bisulfite combination
This is a characteristic reaction in the analysis of aldehydes and ketones, as it is given by all aldehydes and most ketones except those sterically hindered.
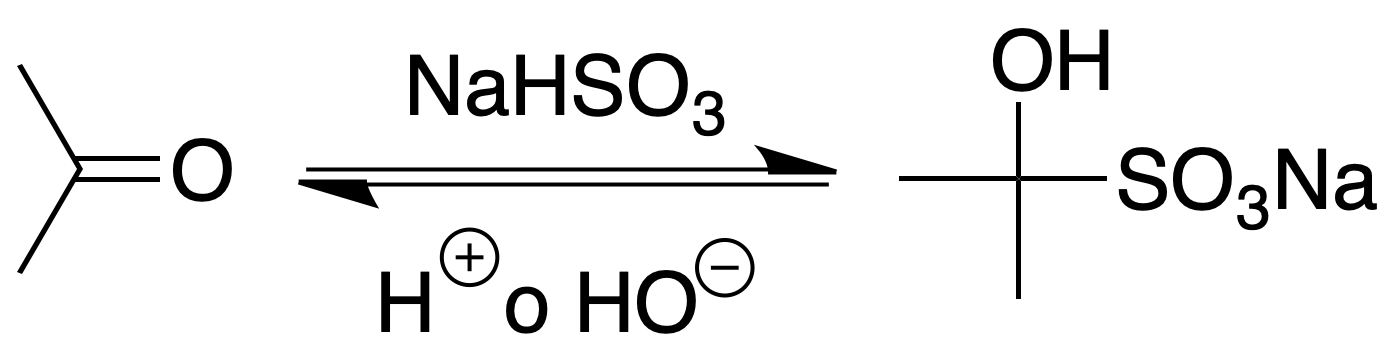
Procedure: Vigorously stir the mixture containing the aldehyde or ketone with a saturated solution of sodium hydrogen sulfite. The temperature will be raised because the addition reaction is exothermic. Collect the crystalline material, wash it with EtOH and ethyl ether and allow to dry. The addition compound can be decomposed with 10% sodium carbonate solution or dilute HCl.
The appearance of a precipitate shows the presence of carbonyl group.
2,4-dinitrophenylhydrazone formation
Aldehydes and ketones are identified by the formation of 2,4-dinitrophenylhydrazones by reaction with 2,4-phenylhydrazine, obtaining a precipitate. If the crystalline product is yellow, it is indicative of a saturated carbonyl compound, if an orange precipitate is obtained it indicates the presence of an α,β-unsaturated system and if what is obtained is a red precipitate it denotes the presence of a ketone or an aromatic aldehyde.
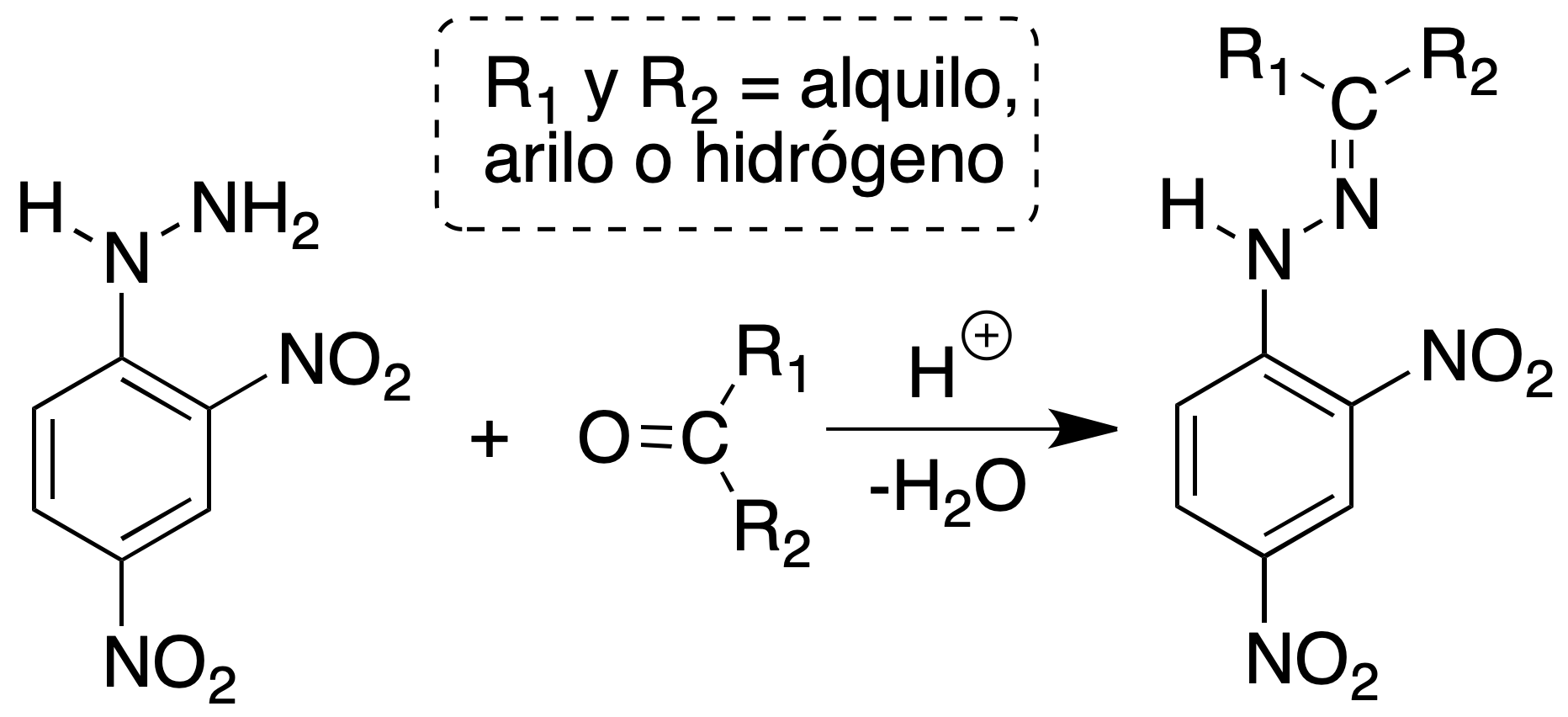
Preparation of the reagent: Dissolve 3 g of 2,4-dinitrophenylhydrazine in 15 ml of concentrated H2SO4. Add this solution, with agitation, on another one of 20 ml of water and 70 ml of EtOH. To mix both solutions and to filter.
Procedure: To a test tube containing 1 ml of reagent add a drop of the carbonyl compound if it is liquid or about 50 mg if it is solid dissolved in EtOH. Shake the mixture vigorously. If a solid is not formed immediately, to let rest 15 min.
The formation of a yellow to red precipitate is considered a positive result. Determine the melting point taking into account the possibility that it is 2,4-dinitrophenylhydrazine (melting point = 198 ºC).
Differences between aldehydes and ketones
Tollens’ reagent
The reagent is an ammoniacal solution of AgOH which is prepared at the time of use.
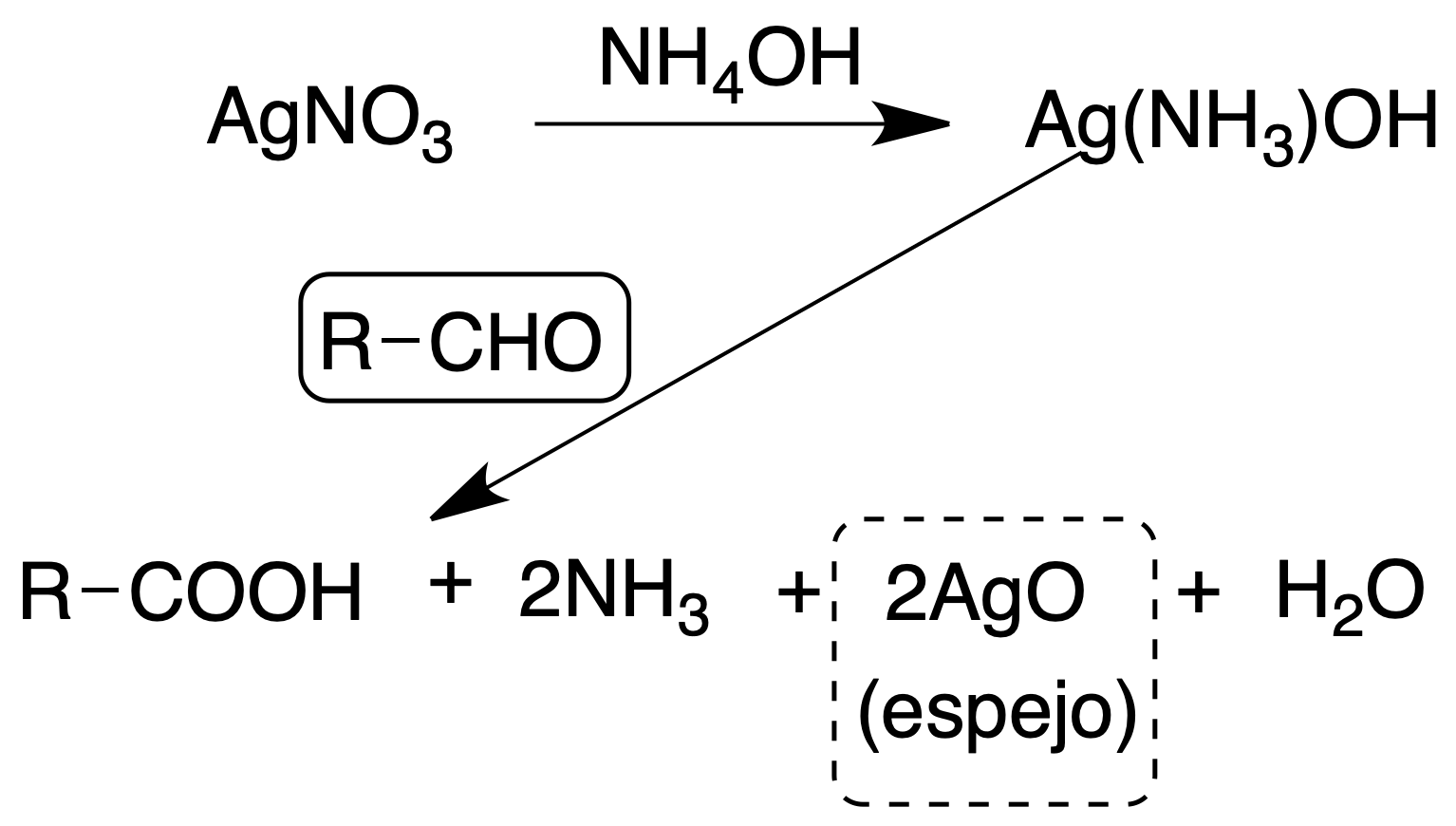
Ketones do not give this reaction, except hydroxyketones and 1,2-dicetones which are reductive and some nitrogenous compounds such as hydrazines, hydroxylamines, aminophenols, which differ from these easily.
Procedure: Prepare two solutions.
- Dissolution A: Dissolve 3 g of silver nitrate in 30 ml of water.
- Solution B: A solution of NaOH at 10 %.
When the reagent is needed, to mix in a clean test tube 1 ml of each of both solutions (A and B) and to add drop by drop a diluted solution of ammonia until total dissolution of the silver oxide. Add a few drops of a dilute solution of the compound to the previous mixture. In a positive test, the silver is deposited in mirror form on the walls of the tube either cold or after heating in a hot water bath. To wash the tube and remove the deposited silver, do so with dilute HNO3.
Fehling’s reagent
Differences of ketones with respect to aldehydes
The most commonly used reaction is the iodoform reaction. It is used for the identification of methyl ketones.
It is necessary to indicate that in addition to these, those substances that can give methylketones by oxidation, in addition to EtOH and acetaldehyde, also give positive results.
Iodoform test for methyl ketones
It consists of the cleavage of the carbonyl compound by the methyl carbonyl bond and subsequent oxidation to carboxylic acid.
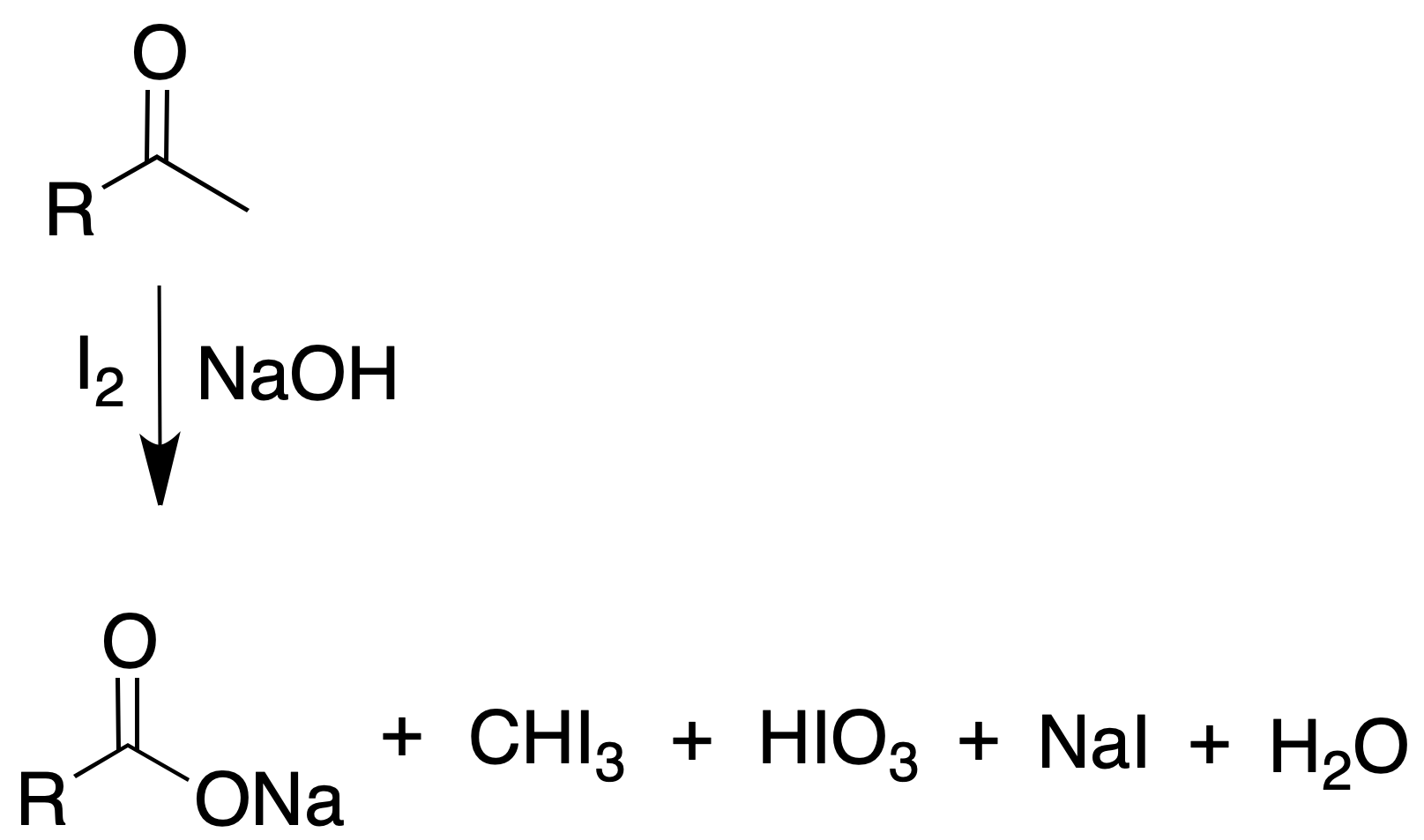
Procedure: The reagent is prepared by dissolving 20 g of potassium iodide and 10 g of iodine in 100 ml of water. Dissolve 5 or 6 drops of the compound (approx. 100 mg) in 2 ml of water. Add dioxane if necessary to dissolve the sample.
| DANGER! “Carry out the test in a fume hood and wearing gloves.“ |
Add 1 ml of 10 % NaOH and the reagent I2 / I–, dropwise and with agitation, until the dark color of the iodine persists. Allow to stand for a few minutes. If precipitate does not appear to heat in a bath to 60 ºC, if the color disappears when heating, to add more reagent, until the color remains.
Then add a few drops of sodium hydroxide solution, dilute with 4 ml of water and allow to stand for 15 min. A positive result will be indicated by the appearance of a yellow precipitate (melting point = 119-121 ºC) with a characteristic medicinal odor.
Characterization of aldehydes and ketones
Phenylhydrazones
Procedure: Place in a test tube 100 mg of carbonyl compound, 4 ml of MeOH and 4 drops of phenylhydrazine. Boil the mixture for 1 min, add a drop of glacial acetic acid and boil again for 3 min.
Add cold water dropwise until a permanent turbidity appears. Cool, collect the crystals and wash them with 1 ml of water containing acetic acid. Recrystallize them from hot MeOH adding water dropwise until cloud point.
2,4-Dinitro- and p-nitro-phenylhydrazones
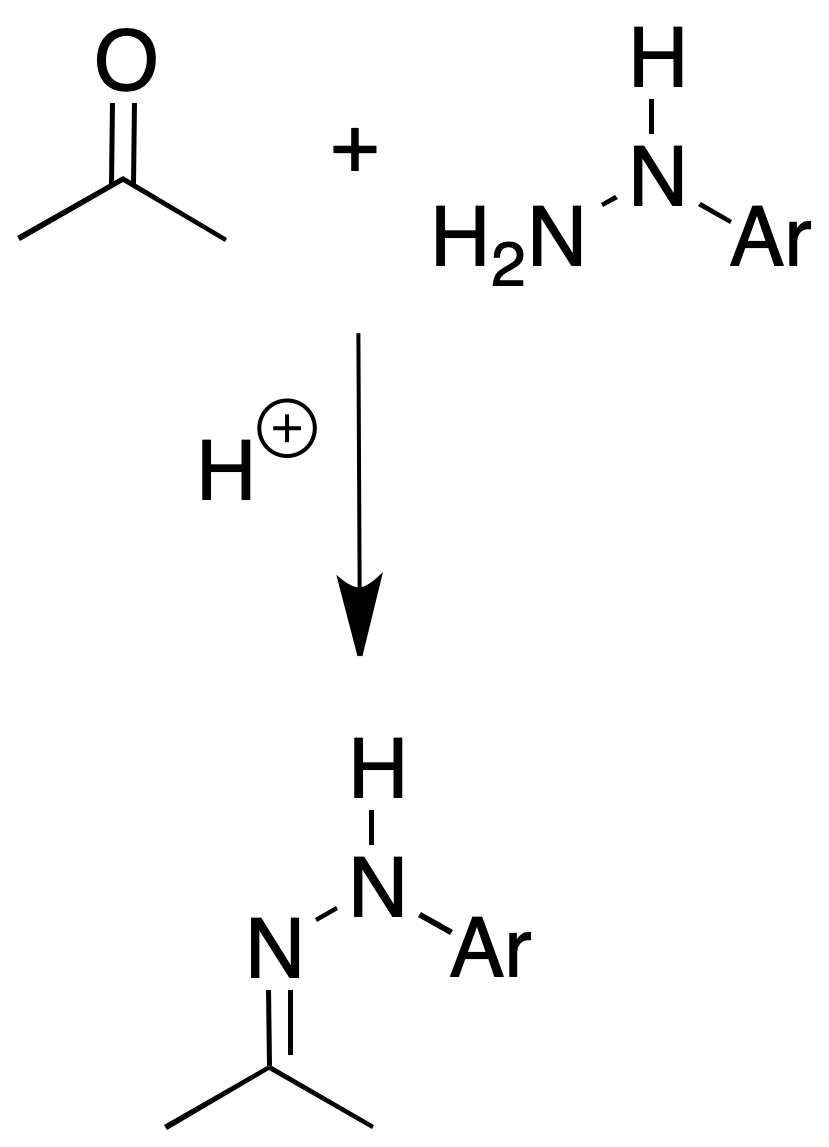
Procedure: Place 100 mg of the nitrophenylhydrazine in a test tube or Erlenmeyer flask with 10 ml of MeOH. Add 5 drops of concentrated HCl and heat if necessary to complete dissolution. Dissolve about 100 mg of the compound in 1 ml MeOH and add to the reagent.
Heat the mixture on a steam bath for 2 min and allow to stand for 20-30 min. To ensure crystallization it is advisable to add water until persistent turbidity.
Semicarbazones
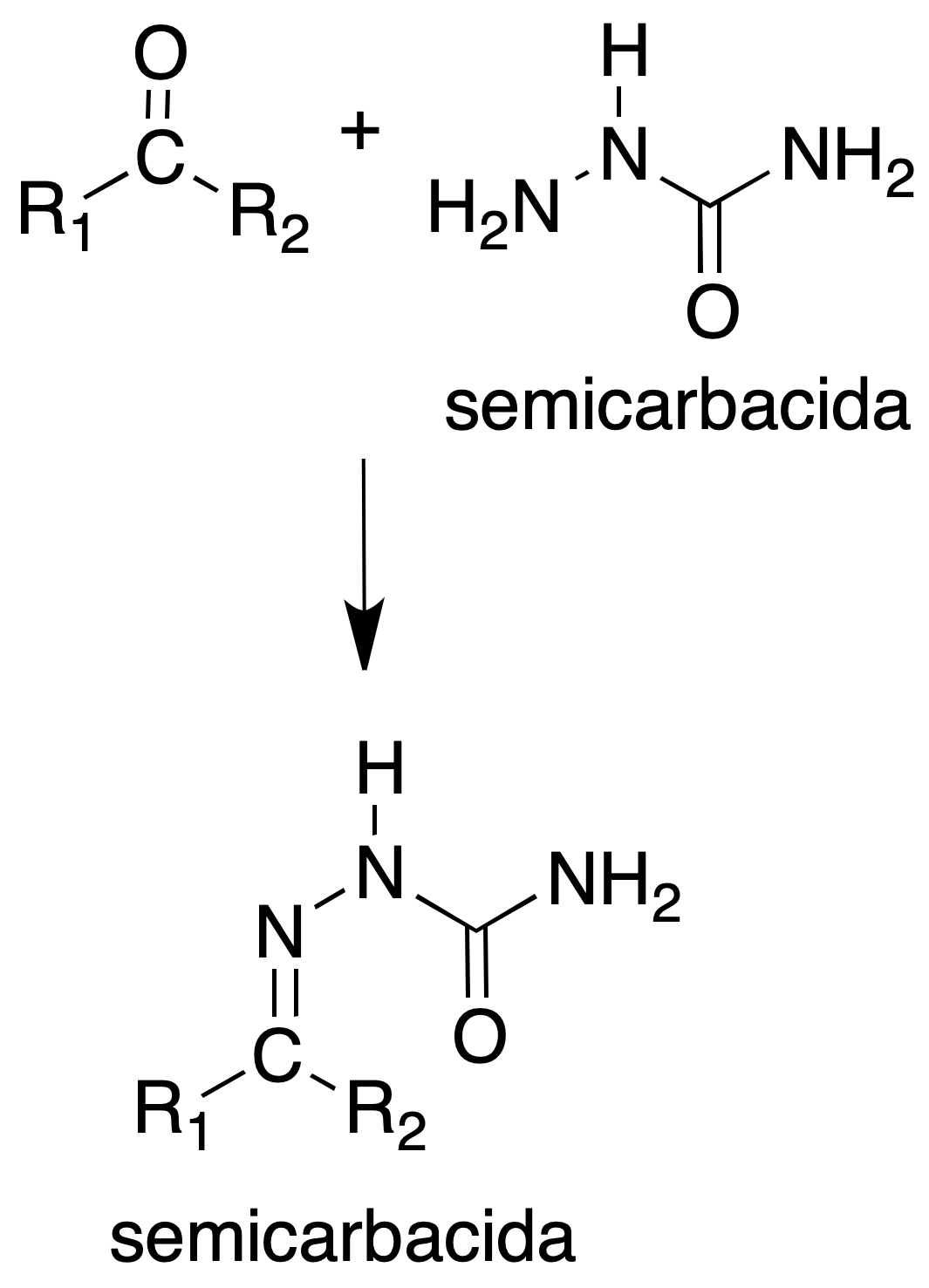
Procedure: In a test tube place 100 mg of semicarbazide hydrochloride, 150 mg of sodium acetate, 1 ml of water and 1 ml of EtOH. Add 100 mg of carbonyl compound. If the mixture is cloudy add more EtOH until it becomes clear. Shake the mixture for a few minutes and let it stand.
The reaction can be accelerated by heating on a steam bath for 10 min and then cooling on an ice bath. The crystals are separated by filtration and washed with cold water. They are usually recrystallized from MeOH or EtOH, sometimes with the aid of water.
Oximes
They can be obtained as the semicarbazones with heating. But also as follows:
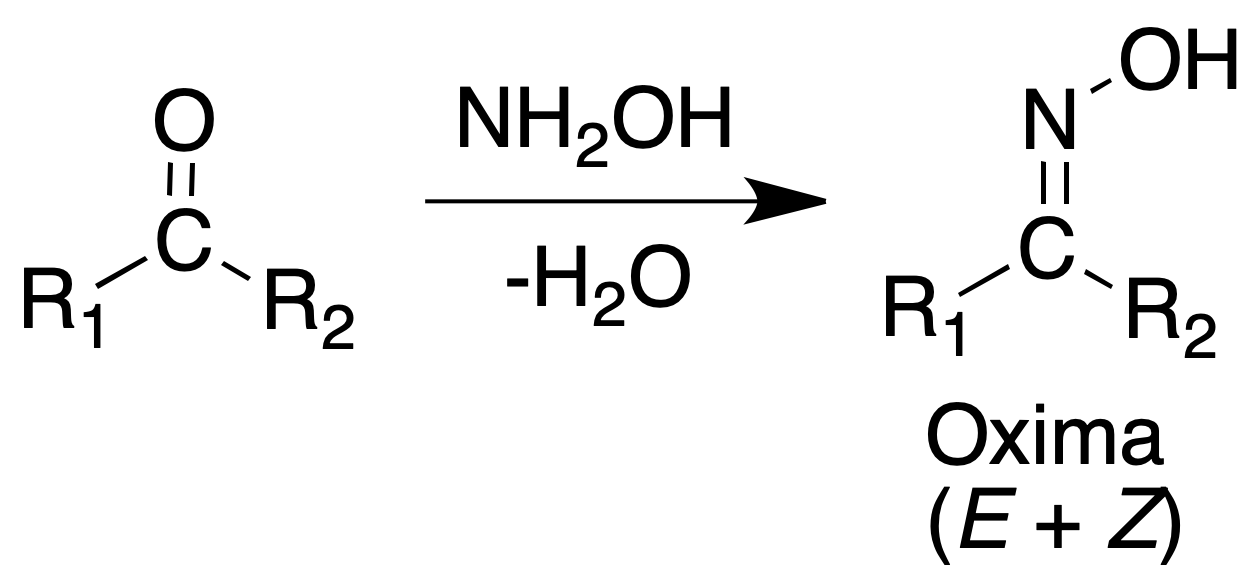
Procedure: Heat at reflux a mixture of 100 mg aldehyde or ketone, 100 mg hydroxylamine hydrochloride, 2 ml EtOH and 0.5 ml pyridine in a water bath for 15 to 60 min. Eliminate the solvent under reduced pressure.
Add several milliliters of cold water and triturate thoroughly. Collect the oxime and recrystallize it from EtOH, EtOH/H2O or benzene.