In the analysis of alkyl halides, the presence or absence of halogens in organic compounds can be determined by the Beilstein test, consisting of the formation of copper halides that give the flame a green or blue coloration, or by the analysis of the filtrate obtained after fusion with sodium.
In order to classify the different types of halogenated compounds, the following two tests are used in the analysis of alkyl halides:
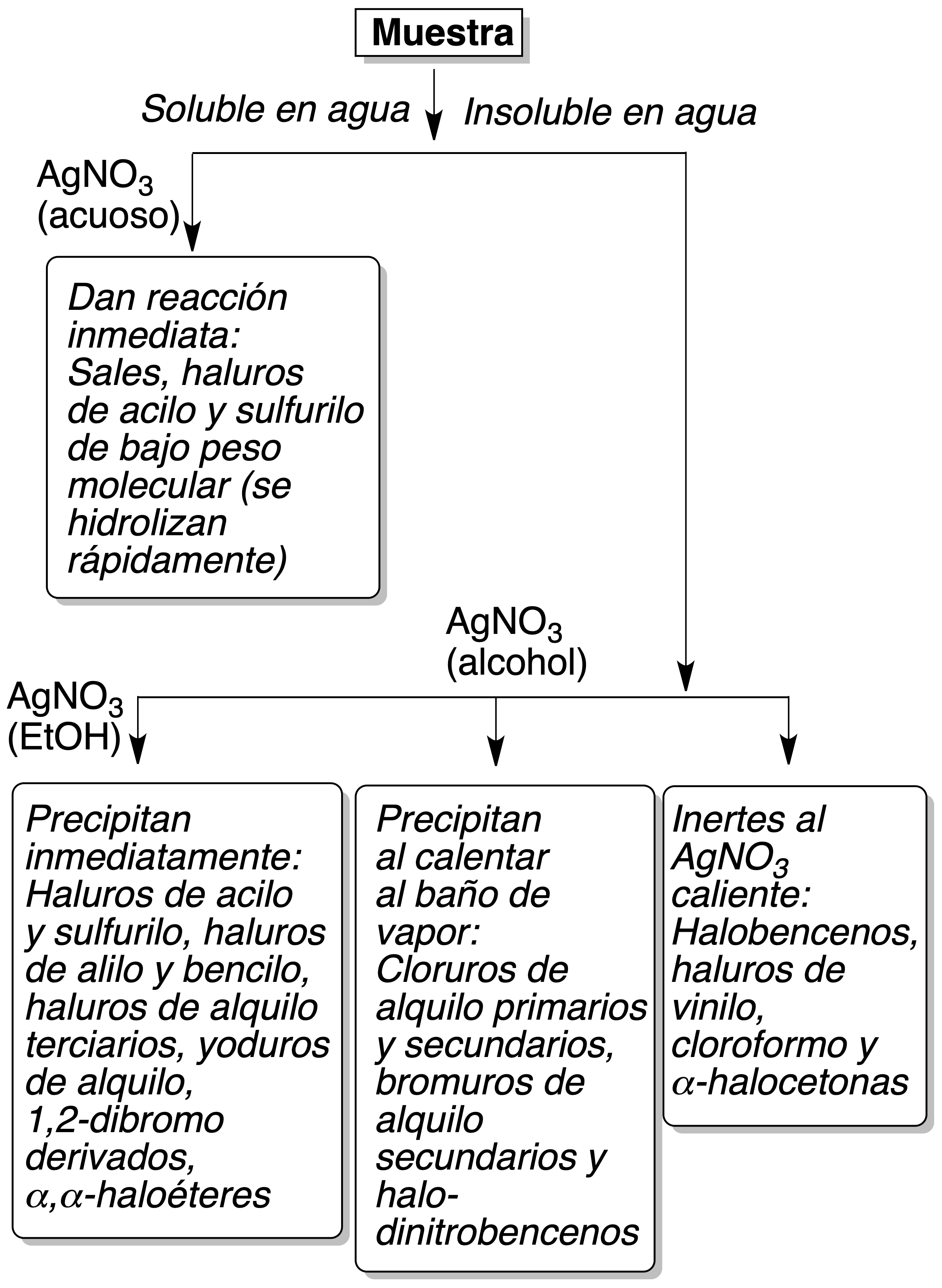
Alcoholic silver nitrate test
Procedure : Add a drop of the halogenated compound to 2 ml of ethanolic silver nitrate at 2 %. If after 5 min no reaction is observed, to heat the solution during several minutes until it boils; observing the color of the formed precipitate; to add two drops of HNO3 to 5 %.
Some organic acids give insoluble silver salts, while silver halides are insoluble in dilute HNO3; usually the silver salts of organic acids are soluble. For water-soluble organic compounds, the test is done with aqueous silver nitrate.
Based on the results obtained, halogenated products can be classified as shown in Figure
Sodium iodide in acetone test
Procedure : Add 2 drops (or 100 mg) of product dissolved in a small amount of acetone to 1 or 2 ml of the reagent (15 g of sodium iodide in 100 ml of acetone) and allow the solution to stand for 3 or 4 min at room temperature.
If no change occurs, heat the test tube at 50 °C for 6 min. Cool to room temperature and note the observed changes.
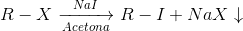
Based on the results we can classify the halides as follows:
- Precipitate in 3 min at room temperature: Primary bromides, acyl halides, allyl halides, (α)-halocarbonyl compounds and R-CCl3.
- Precipitate only when heated at 50 ºC for 6 min: primary and secondary chlorides, secondary and tertiary bromides and bromoform.
- Do not react at 50 ºC for 6 min: vinyl and aryl halides, geminal polychlorinated compounds.
- React to give precipitate and also release iodine (brown color): vecyl halides, sulfonyl halides, triphenylmethyl halides.
Characterization of alkyl halides (S-alkylthiouronium picrates)
They are a group of solid derivatives easily obtained from primary and secondary alkyl halides (tertiary ones do not react) according to the following procedure:
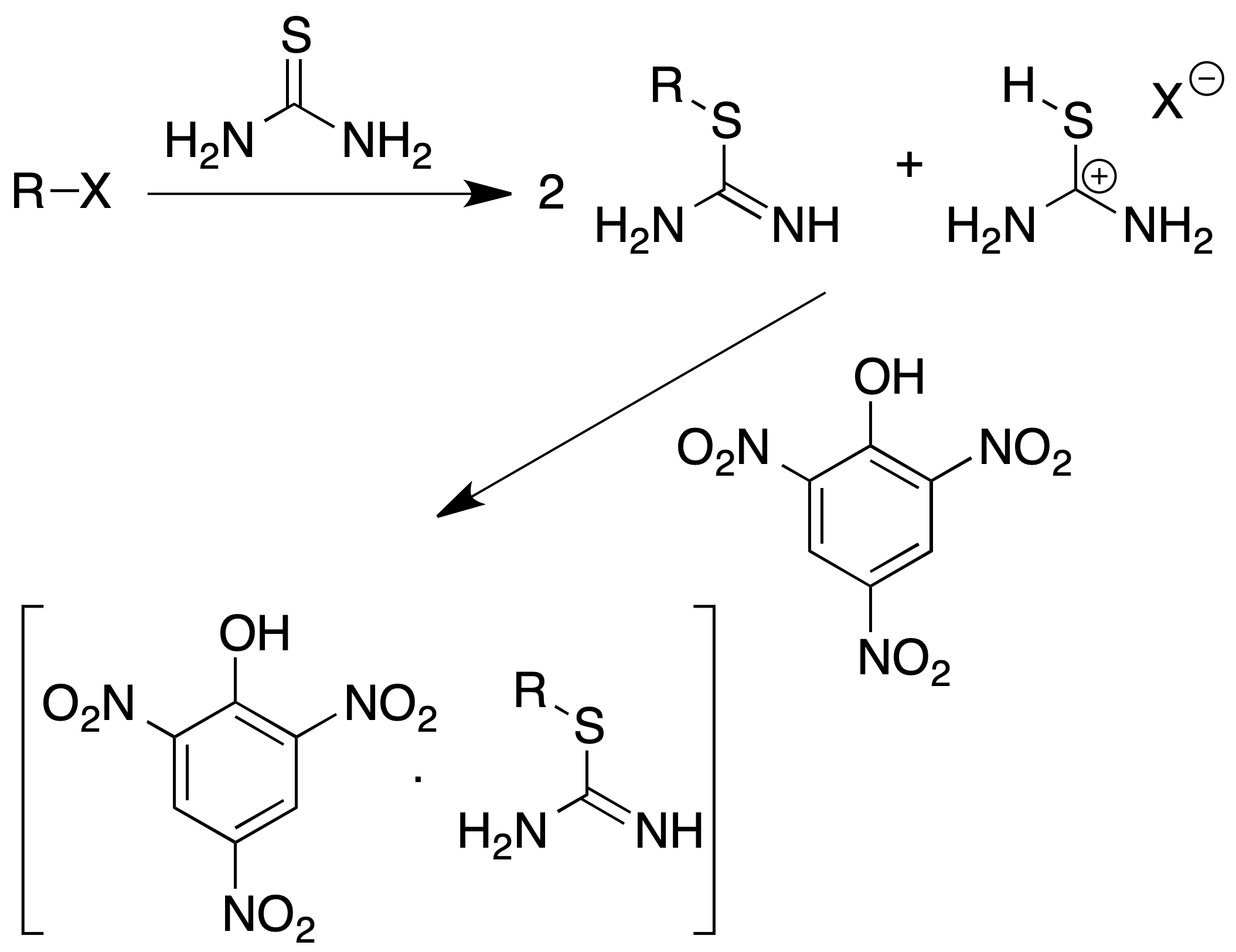
Procedure : Alkyl halide (1 mmol) and thiourea (150 mg) are dissolved in 5 ml of ethylene glycol in a flask fitted with a condenser (reflux) and the mixture is heated for 30 min.
The primary alkyl iodides require heating to a temperature of approx. 65 ºC, the other halides require temperatures of approx. 120 ºC.
Then add 1 ml of saturated solution of picric acid in EtOH and heat for 15 min more. Cool the reaction mixture and add 5 ml of cold water. Allow to stand in an ice bath. Filter the precipitate.
Characterization of aryl halides
The usual procedure consists of nitration, as indicated for simple aromatic hydrocarbons.