Quinones are colored crystalline compounds (most of them are yellow) with a pungent odor. They show in the IR spectrum a carbonyl band near 1670 cm-1.
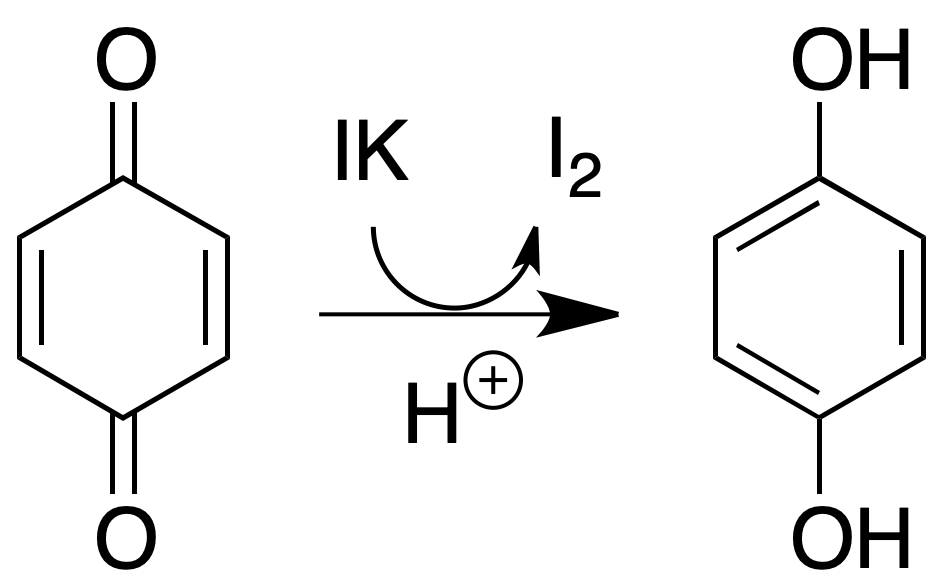
Iodide test
Most quinones release iodine from acidified potassium iodide solutions.
Oximes and semicarbazones
Quinones often form oximes and semicarbazones that often do not have the usual structure.
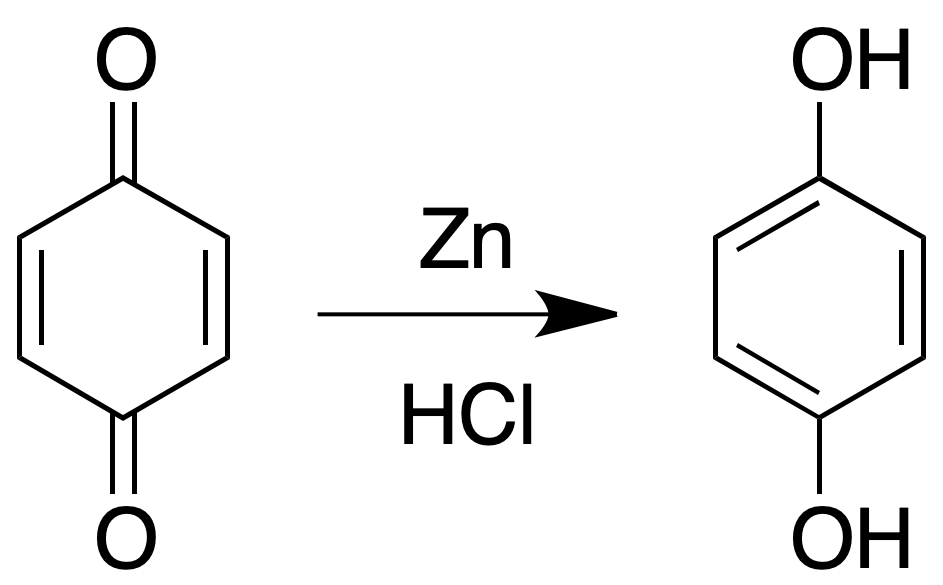
Reduction to dihydroxybenzenes (diphenols)
Suspend the quinone in dilute HCl and add a “pinch” of zinc powder. When the solution becomes colorless, neutralize it with sodium bicarbonate and extract the dihydroxybenzene with ether. Evaporate the solvent and identify it.
Catechol (o-dihydroxybenzene) and hydroquinone (p-dihydroxybenzene) reduce the Tollens’ reagent when cold and the Fehling’s reagent when hot (see Fehling’s test).
Resorcinol (m-dihydroxybenzene) reduces both in the hot state. Pyrogallol (1,2,3-trihydroxybenzene) also reduces silver salts to metallic silver.
Quinoxaline formation
This test is applicable for o-quinones.
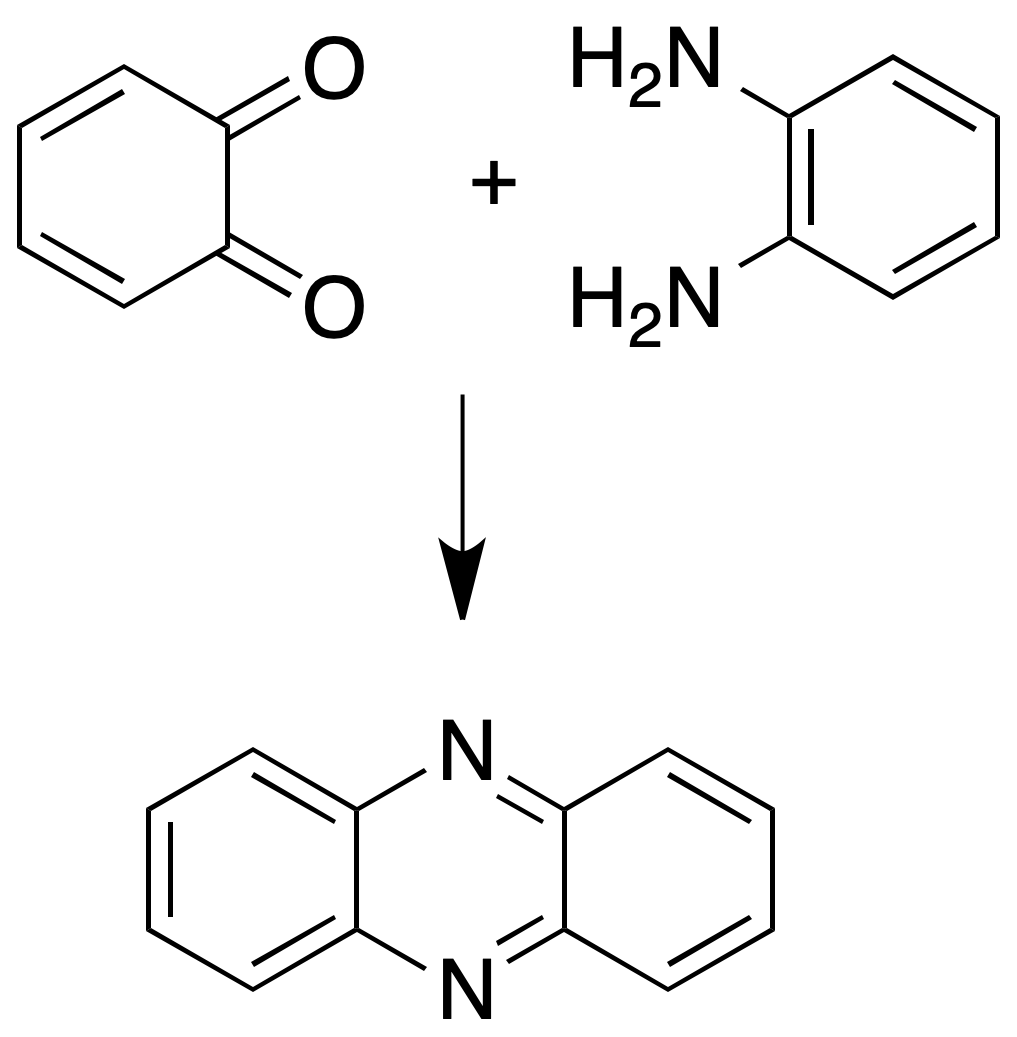
Dissolve the o-quinone in alcohol or glacial acetic acid and add an equivalent amount of o-phenylenediamine in alcohol. Heat the mixture on a steam bath for 15-20 min, cool and dilute with water to crystallize. Recrystallize in EtOH/H2O.