What is an azeotrope?
An azeotrope is a mixture of two or more components whose proportions are such that the vapor produced by partial evaporation has the same composition as the liquid. Therefore, they behave in distillation as if they were a pure compound (see Table 1).
When a mixture is at the azeotropic point (azeotropic mixture), it cannot be distilled or separated into its components.
In azeotropic distillation, the vapor and liquid phases of the mixture have the same composition as the original mixture, making it difficult to separate the components through simple distillation.
One common method of azeotropic distillation is called extractive distillation, in which a third component, known as an entrainer, is added to the mixture. The entrainer forms an azeotrope with one of the components of the mixture, allowing it to be separated through distillation. The effect achieved is to change the volatility of one of the components of the azeotrope to a greater extent than the other, allowing the separation to take place. The entrainer can then be separated from the desired component through an additional distillation step.
Another method of azeotropic distillation is called pressure-swing distillation, in which the pressure of the system is changed during the distillation process. This allows for the separation of the components of the mixture due to the differences in their vapor pressures.
Examples of azeotropes
There are a multitude of combinations between two solvents that result in an azeotropic mixture, and Table 1 summarizes the most common ones.
| Component A | Component B | Azeotrope | ||
| B.p. (ºC) | % (weight) | B.p. (ºC) | % (weight) | B.p. (ºC) |
| H2O (100) | 1.3 | Diethyl ether (34.5) | 98.7 | 34.2 |
| H2O (100) | 1.4 | Pentane (36.1) | 98.6 | 34.6 |
| MeOH (64.7) | 12.1 | Acetone (56.1) | 87.9 | 55.5 |
| MeOH (64.7) | 72.5 | Toluene (110.7) | 27.5 | 63.5 |
| EtOH (78.3) | 68.0 | Toluene (110.7) | 32.0 | 76.7 |
| H2O (100) | 13.5 | Toluene (110.7) | 86.5 | 84.1 |
What is azeotropic distillation?
Azeotropic distillation is a useful procedure for removing a liquid from a reaction crude by co-distillation with an immiscible organic solvent.
This technique is often used in equilibrium reactions where water is generated as a by-product of the reaction. The removal of water will shift the equilibrium of the reaction to the product side.
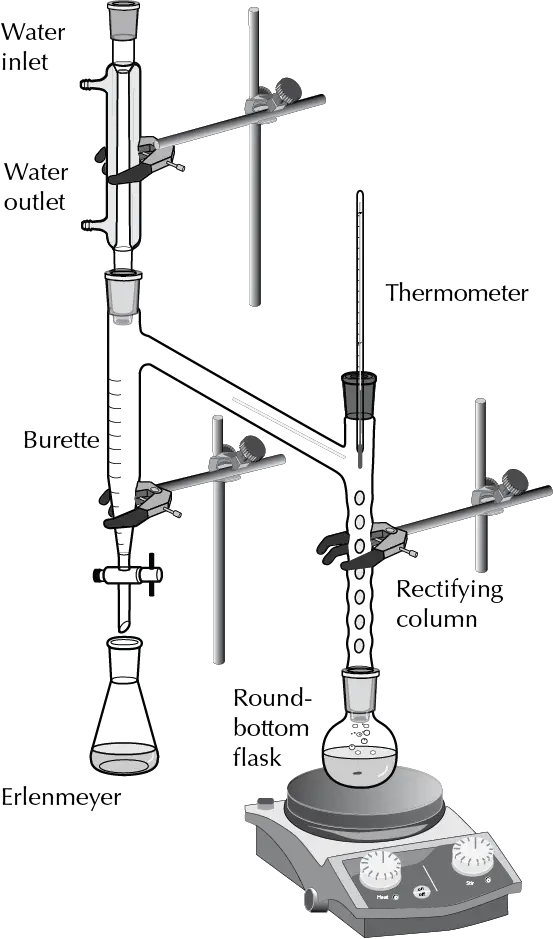
Azeotrope water/toluene separation
If the reaction is carried out for example with toluene, which is less dense than water, the vapor in the reflux condenser will consist of an azeotropic mixture of toluene and water. When this mixture condenses, it falls into the so-called Dean-Stark apparatus (Clevenger apparatus), forming two phases, the upper layer consisting of toluene and the lower one of water.
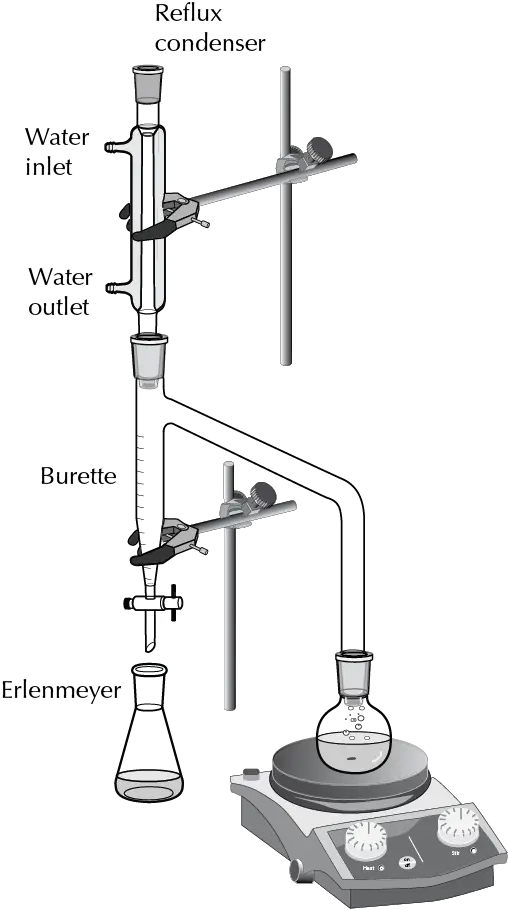
When the liquid level in the Dean-Stark trap reaches the top of the side arm, the toluene flows back into the reaction flask. Water can be removed through a tap at the bottom of the Dean-Stark trap.
The Dean-Stark apparatus was invented in 1920 by U.S. Chemists Ernest Woodward Dean (1888-1959) and David Dewey Stark (1893-1979), applied to the determination of water content in petroleum.
The Dean-Stark apparatus is used in combination with a glass condenser and a reactor for continuous removal of the water that is produced during a chemical reaction performed at reflux temperature.
Application examples
The azeotropic distillation is also used in dehydration equilibrium reactions. Because some of them present not only an equilibrium that reverses the reaction when water appears, but the process is fast. Examples of this type of reactions are the formation of dioxolanes from aldehydes as shown in the scheme.
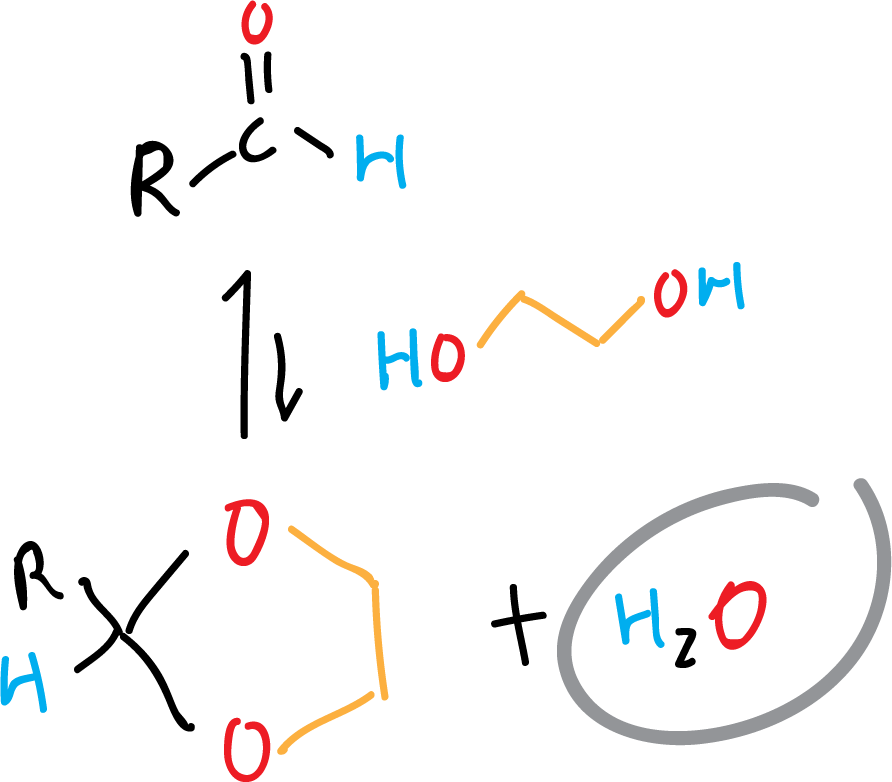
When water is removed from the reaction by azeotropic distillation, the equilibrium shifts favorably towards the formation of the products.
Breaking azeotropes
Azeotropes of low boiling point, cannot be completely purified by distillation, to obtain the most volatile component. Alternatively, the pure most volatile component can be separated by breaking the azeotrope.
This is achieved by a method other than distillation, using molecular sieves. The use of molecular sieves is used to break the EtOH/H2O (96%) azeotrope and obtain the anhydrous alcohol, with the water remaining absorbed in the molecular sieves. The sieves can be reused later by dehydration using an oven.
Summary
Azeotropic distillation is a special technique in which another component is added to break intermolecular interactions, resulting in a hetereogenous mixture (as it produces two phases with immiscible liquids and new azeotrope). For example, toluene (better than benzene which is carcinogenic) can be added to an azeotropic mixture of ethanol/water, to separate the water from the azeotrope.
The Dean-Stark apparatus can be used to perform azeotropic distillations in the laboratory, and is also particularly suitable for separating water from a reaction, e.g. in toluene/water mixtures.
Azeotropic distillation is commonly used in the chemical industry to separate mixtures of liquids that cannot be separated through simple distillation. It is also used in the production of high-purity chemicals and in the purification of liquids.
Overall, azeotropic distillation is a useful technique for separating mixtures of liquids that have a constant boiling point. It allows for the separation of components that would otherwise be difficult to separate through simple distillation.
Video on azeotropic distillation
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145