What are azoles?
The azoles are five-membered cyclic organic compounds having one or more non-carbon atoms in the ring, at least one of the heteroatoms being nitrogen. This is the largest and most diverse group of aromatic heterocycles. They are derived from furan, thiophene and pyrrole, by replacement of one or more CH groups by sp2-hybridized nitrogens.
It is the variation in the number and positions of the nitrogen atoms that gives rise to the great structural diversity of this group of heterocycles. The preferred names for these heterocycles end in azole and are therefore called azoles.
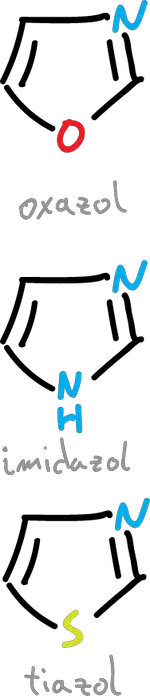
When the substitution of a CH for a nitrogen is made at the C3 position the structures (oxazole, thiazole e imidazole) would be as follows.
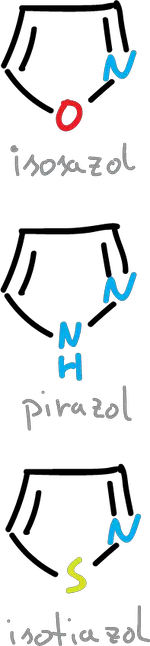
If the nitrogen substitution is made at the C2 position we are left with the following structures (isoxazole, isothiazole and pyrazole).
The triazoles and tetrazoles, with 3 and 4 nitrogen atoms, respectively, are named systematically (see nomenclature).
The presence of the additional nitrogen atoms in the rings has relevant effects on the properties of these compounds.
These nitrogen atoms contain unshared electron pairs in the ring plane, which do not participate in the π-system. Therefore, they allow the azoles to behave as bases and have some degree of nucleophilic character.
The imidazole is a strong base, but the other azoles are weak bases.
Generally speaking, the basicity decreases as the number of nitrogen atoms increases due to the inductive and electron-attracting effect of the nitrogen atoms. Those containing oxygen are the least basic due to the inductive effect of oxygen.
These heterocycles are considered as resonance hybrids of various contributing structures:
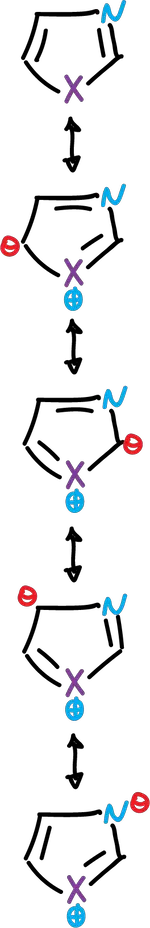
Each structure does not contribute equally to the resonance hybrid. Then, the carbon atoms of the azoles are not equivalent in terms of electron density π, and this effect is reflected in their chemical behavior.
Another important effect of proton mobility is that N-unsaturated diazoles, triazoles and tetrazoles exist in tautomeric forms interrelated by intramolecular proton transfer.
Normally, this transfer is too fast to allow isolation of the individual structures, as illustrated in the examples for pyrazole and imidazole.
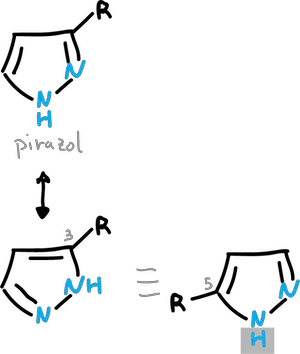
Because of this rapid proton transfer, there is no separate C3– and C5-substituted pyrazoles.
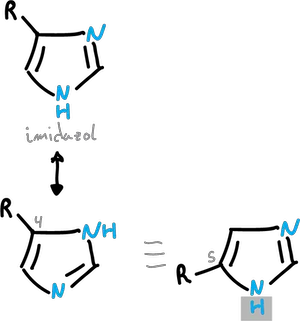
Similarly, imidazoles substituted at C4 and C5 interconvert too rapidly to allow their individual isolation.However, tautomers can exist in solution with predominance of a particular form.
For example, in the case of nitro imidazole where the equilibrium shifts towards the C4 derivative.
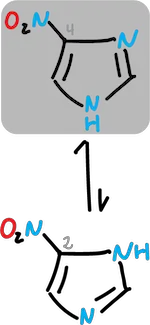
Imidazoles
Pyrazoles, triazoles and tetrazoles
Benzodiazoles and benzotriazoles
There are three ring heterocyclic systems corresponding to benzofused diazoles or triazoles: indazole, benzimidazole and benzotriazole.
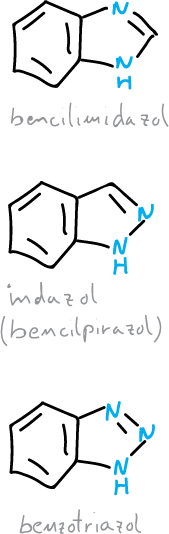
The benzimidazole is part of the structure of vitamin B12, there are also several benzimidazoles that are used as drugs.
Several imidazoles and benzotriazoles are used as optical brightening agents and as light stabilizers for plastics.
The benzotriazole is the strongest acid of the three heterocycles (pKa = 8.2) and the benzimidazole is the strongest base. Thus, the benzimidazolium cation has a pKa of 5.5.
Synthesis
By cyclization reactions starting from 1,2-substituted benzenes of appropriate type. For example, benzene 1,2-diamine with carboxylic acid derivatives.
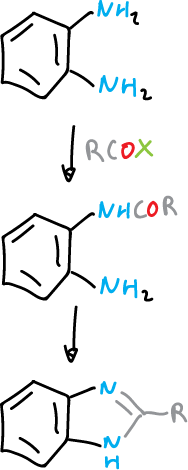
Benzotriazoles are obtained from the same type of reagents by diazotization and diazonium salt cyclization.
On the other hand, imidazoles, analogously diazotizing an aniline having an ortho-carbonylated substituent.
Electrophilic substitution reaction
Nitrations, halogenations and other similar reactions are possible.
Benzimidazole and imidazole are preferentially attacked at the C5 position and benzotriazole at C4.
Nucleophilic attack
Benzimidazoles at their C2 position can be attacked by nucleophiles. Alkoxides, amines, thiols and other nucleophiles displace the chloride at the C2 position of the benzimidazole.
Its reactivity against nucleophiles is lower than that of the corresponding benzoxazoles and benzothiazoles.
Alkylation of the imidazole and benzotriazole usually leads to mixtures of substituted derivatives in the N1 and N2positions, in a ratio depending on the alkylating agent.
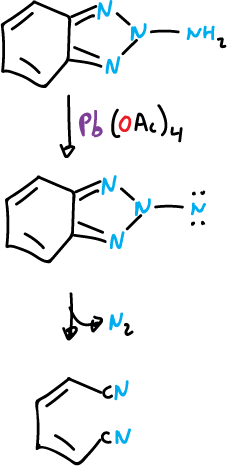
The 1- and 2-amino benzotriazole are rapidly oxidized by lead(IV) acetate, Pb(OAc)4, and in both cases the ring system is opened.
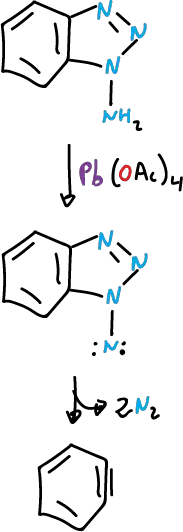
The opening mode of the rings is different.
The benzotriazoles substituted at the N1 position, like the monocyclic 1,2,3-triazoles, can lose nitrogen by thermolysis (Δ) or photolysis (hν).
In the case of arylbenzotriazoles, the intermediates generated by nitrogen elimination can be cycled back to the ortho-position of the aryl substituent, which leads to the formation of a carbazole derivative.
Graebe-Ullman reaction
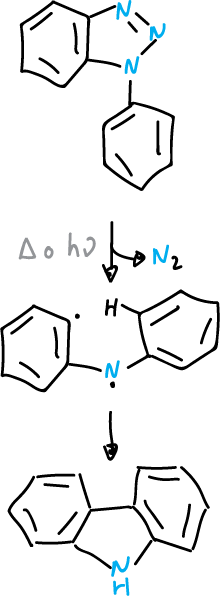
This reaction has been used in the synthesis of alkaloids.
Oxazoles and thiazoles
Isoxazoles, isothiazoles and their benzoderivatives
Oxadiazoles and thiadiazoles
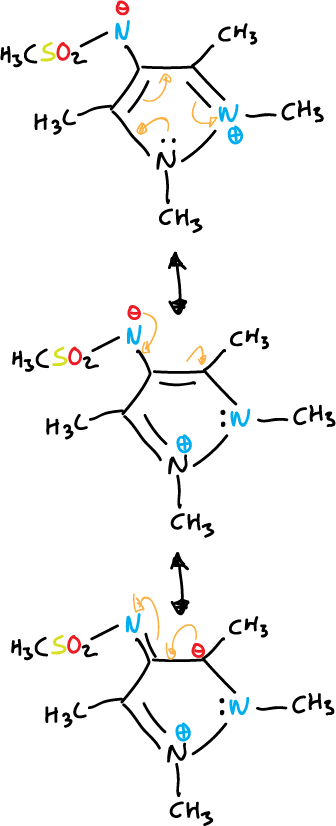
Betaines and mesoionic compounds
Some fully unsaturated 5-membered heterocycles cannot be represented by valence bond structures without using charges.
They are represented by dipolar structures, i.e. they are betaines.
They contain a cyclic arrangement of 5 p orbitals that can be represented as containing six π electrons and a sixth, exocyclic p orbital containing two π electrons.
They can be divided into two general groups:
- Oxides or imides: the exocyclic atom is bound to the system by a heteroatom, as in the case of triazole.
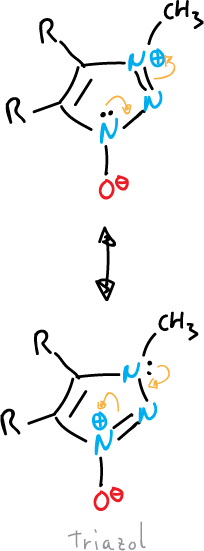
or also, that of pyrazole shown below:
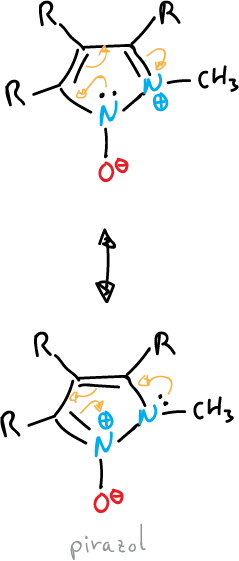
- Mesoionic compounds: the exocyclic atom is bonded through the carbon.
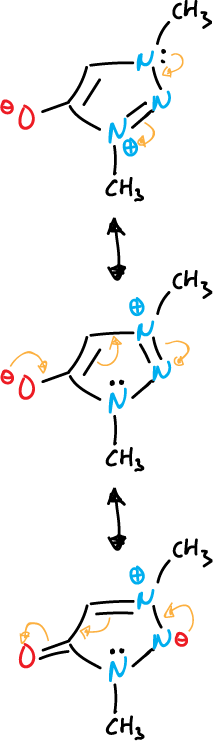
Another example with diazoles is given below.