What are benzo-fused 5-membered heterocycles?
Condensed 5-membered heterocycles are the heteroatoms resulting from the fusion of a benzene ring and a 5-membered heterocycle. The main ones are those where the fusion is performed on the 2-3 bond of the heterocycle.
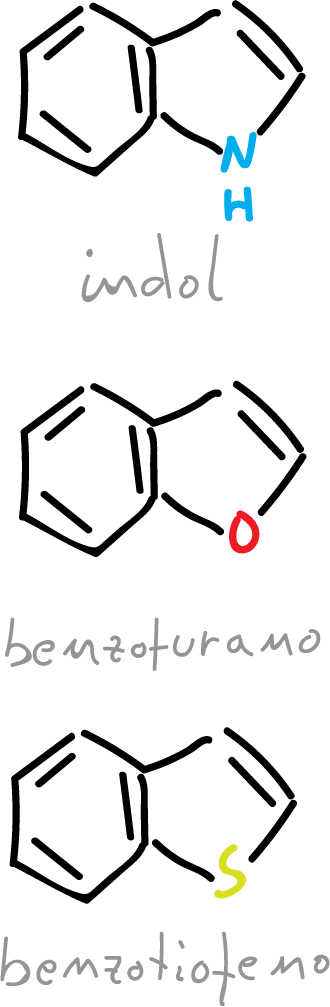
The description of these 3 heterocycles (indole, benzofuran and benzothiophene), from the point of view of molecular orbitals (MO) is similar to furan, thiophene and pyrrole. However, the only additional feature is the distribution of 10 π electrons, in the cyclic system instead of 6 as in the previous case.
It is possible to write several resonant structures, as indicated in the scheme.
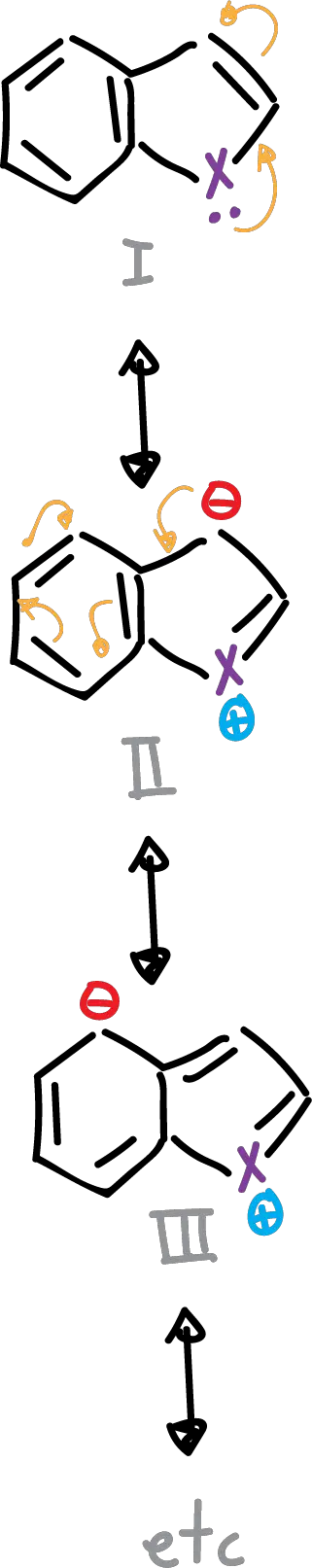
Structures I and II contribute more than the rest, since in the latter (III), the benzenoid resonance is lost and there is a large charge separation.
The most representative family of benzofused heterocycles is composed of the indoles, and therefore they are treated separately in the following link.
Indoles
Other benzo-fused heterocycles
Benzo[b]furanes
Benzofuran forms part of many natural and pharmaceutical products, although to a lesser extent than indole.
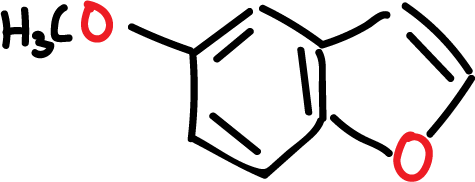
For example, the natural product 5-methoxy derivative exhibits bactericidal properties.
Benzofuran occurs naturally in coal tar, but is prepared on a semi-commercial scale by vapor phase dehydrogenation and subsequent cyclization of the compound 2-ethyl phenol.
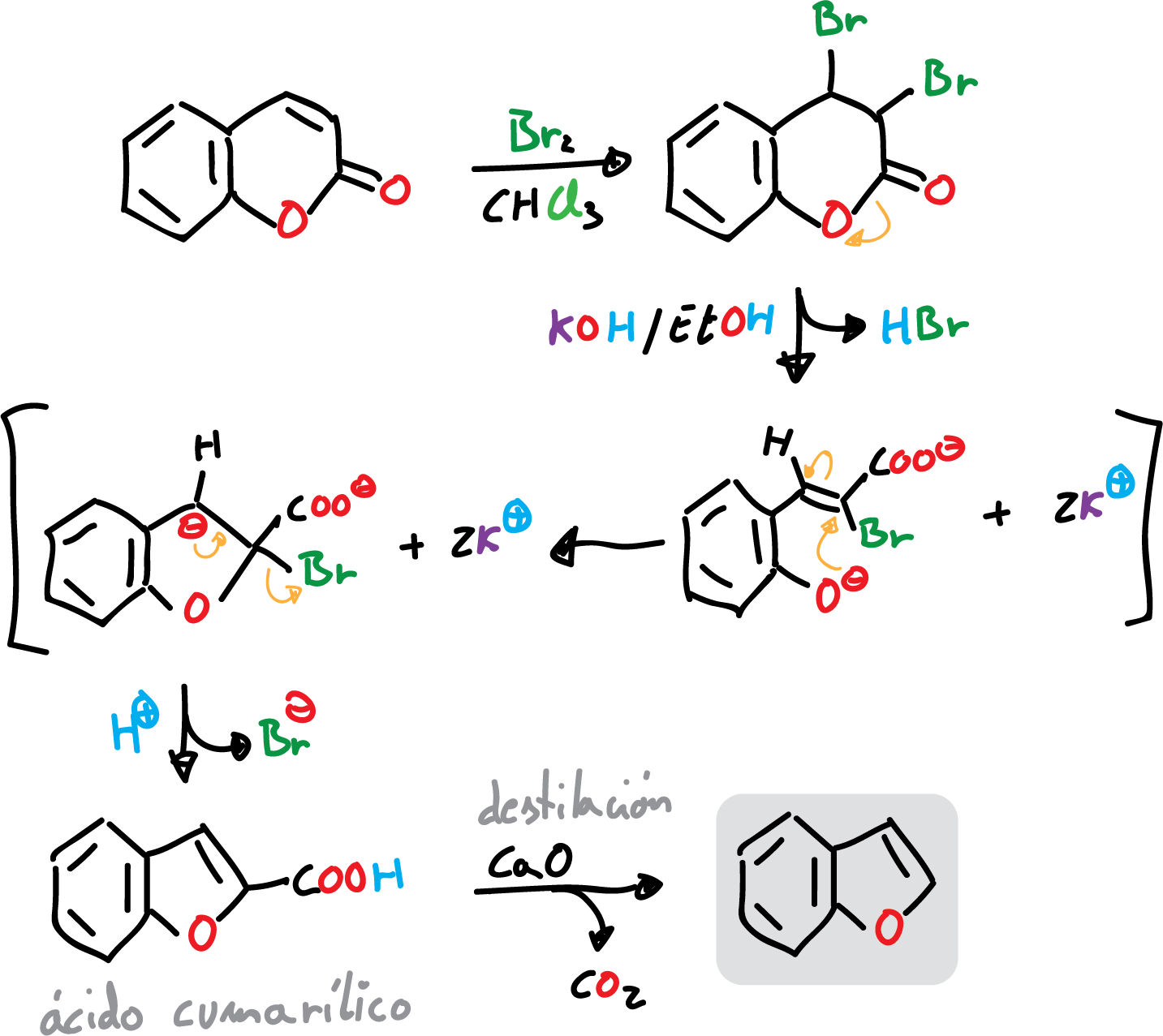
The classical synthesis of benzofuran is carried out by bromination of coumarin, or a derivative thereof. This reaction is followed by treatment of the resulting dibromide with base and decarboxylation of coumarilic acid, as indicated in the scheme.
The alkyl benzofurans and substituted benzofurans are prepared, in general, by cyclization reactions. This is because the synthesis of the ring is simpler than the electrophilic substitution of the heterocycle.
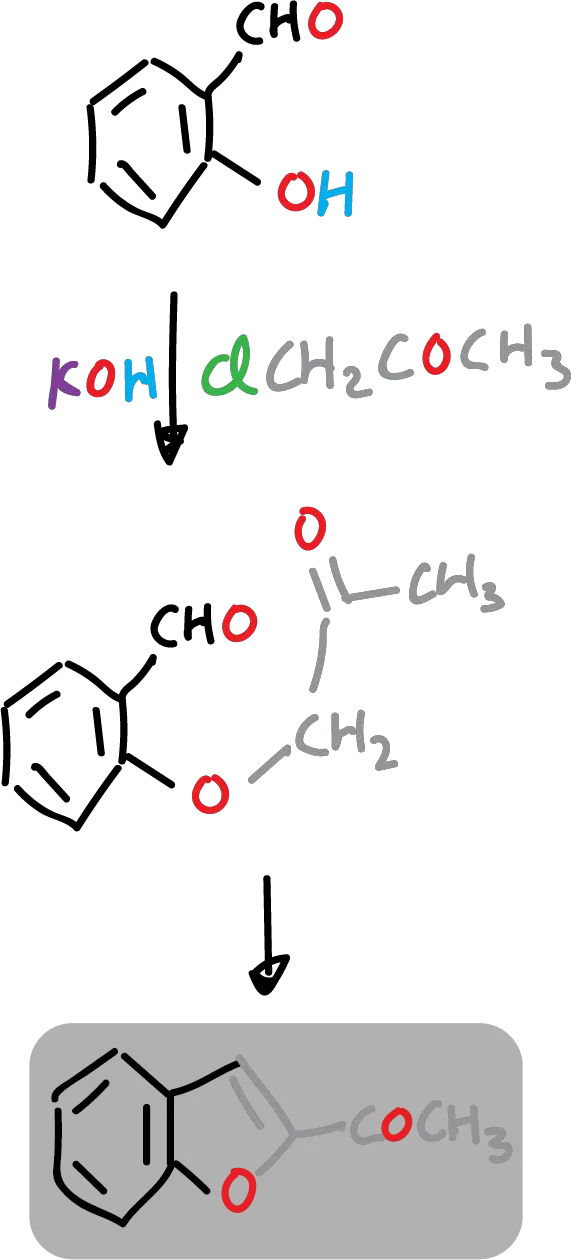
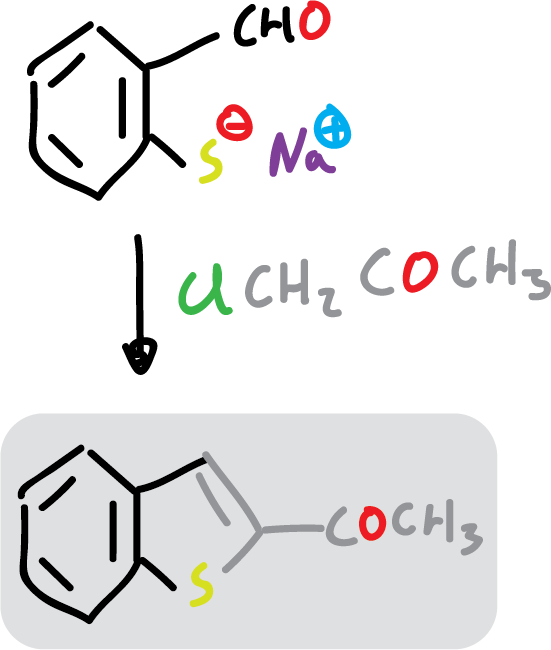
For example: 2-acetylbenzofuran is easily prepared from 2-hydroxybenzaldehyde and chloroacetone (ClCH2COCH3).
As for the reactivity of benzofurans, the aromatic character of the 5-membered ring is weak and the ring is easily opened by the action of oxidizing and reducing agents and polymerizes under the effect of concentrated mineral acids and Lewis acids.
Electrophilic substitution of benzofurans
Benzofuran is less reactive than furan and, under certain conditions, has the characteristics of a vinyl ether.
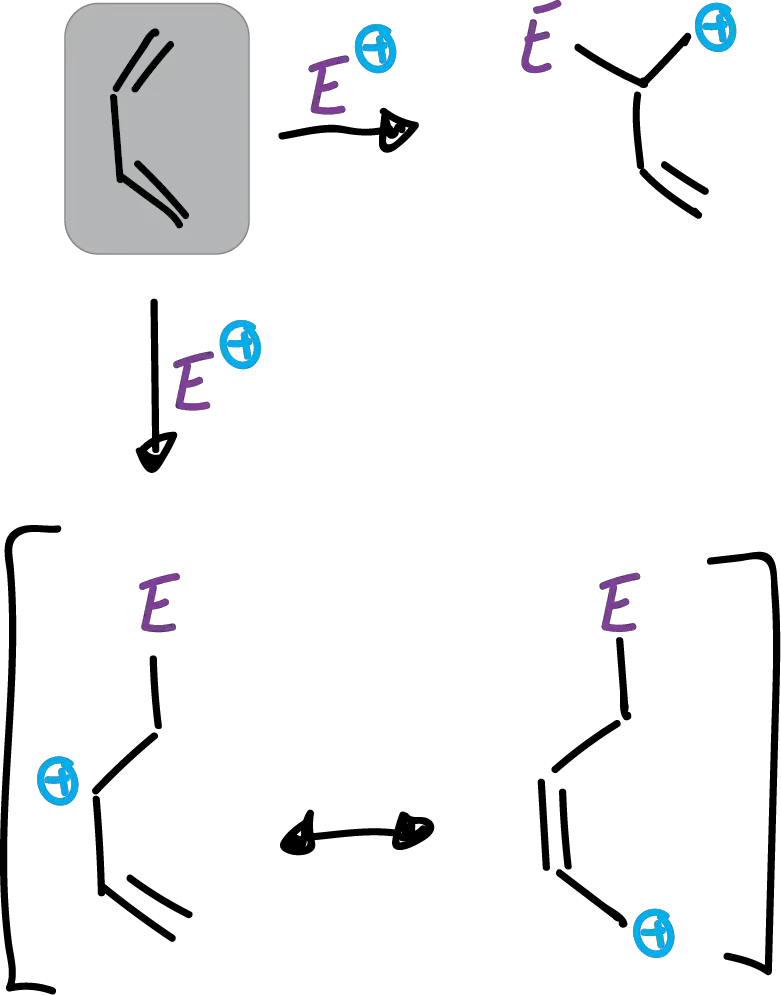
When it undergoes electrophilic substitution it occurs preferentially at the C2 position and not at the C3 position, as was the case for indoles.
This difference in orientation has been attributed to differences in electronegativities between oxygen and nitrogen (O > N> S). which gives rise to what is called the α-effect. Thus, in butadiene the electrophile attacking in the C1 position is favored.
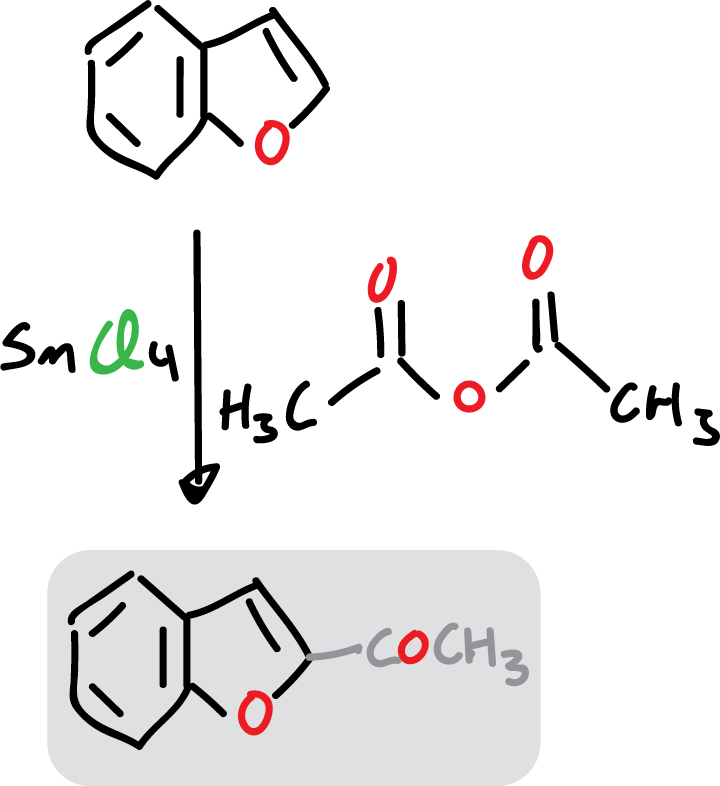
Examples of substitution at C2 occur when benzofuran reacts with stannous chloride (SnCl4) and acetic anhydride.
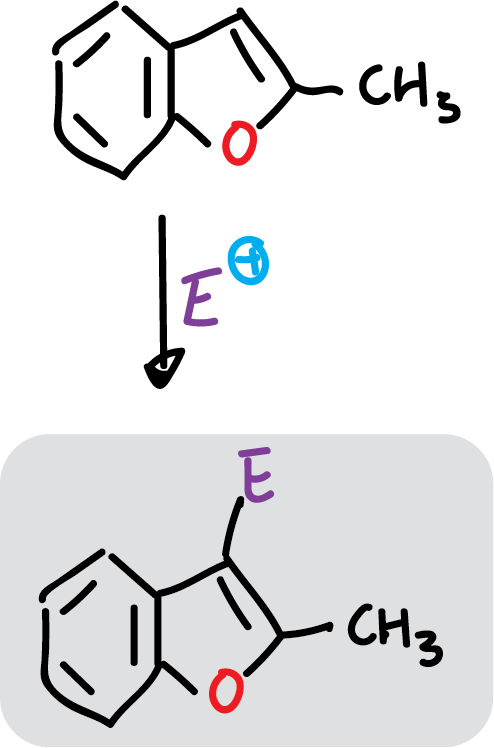
However, when the C2 position of benzofuran is occupied by an electron donor, the substitution occurs at C3.
Moreover, the presence of an electrophilic substituent on the heterocyclic ring disables it for further substitutions, and the subsequent attack would then occur on the benzene ring. Thus, the same would occur as when both positions of the heterocycle were occupied.
On the other hand, sometimes a substituent is displaced from the heterocyclic position of the molecule.
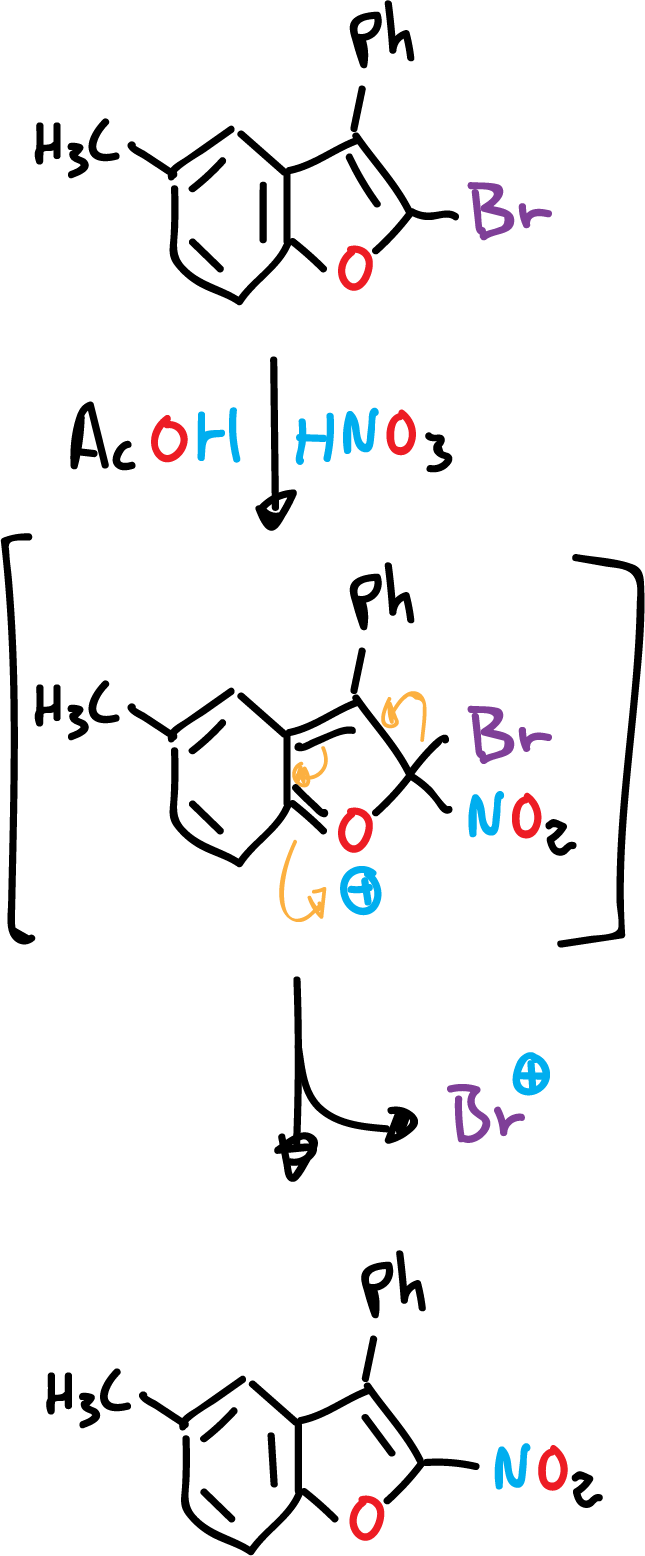
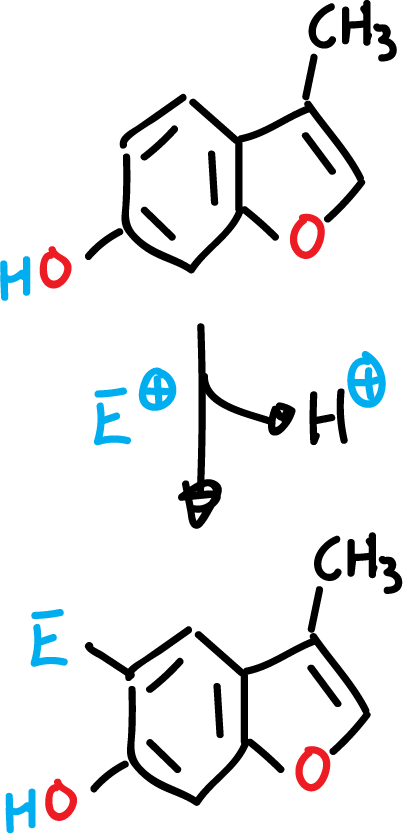
The presence of an amino or hydroxyl function in the benzene ring, for these heterocycles, orient the substitution exclusively in the benzene ring, even if the rest of the positions are empty.
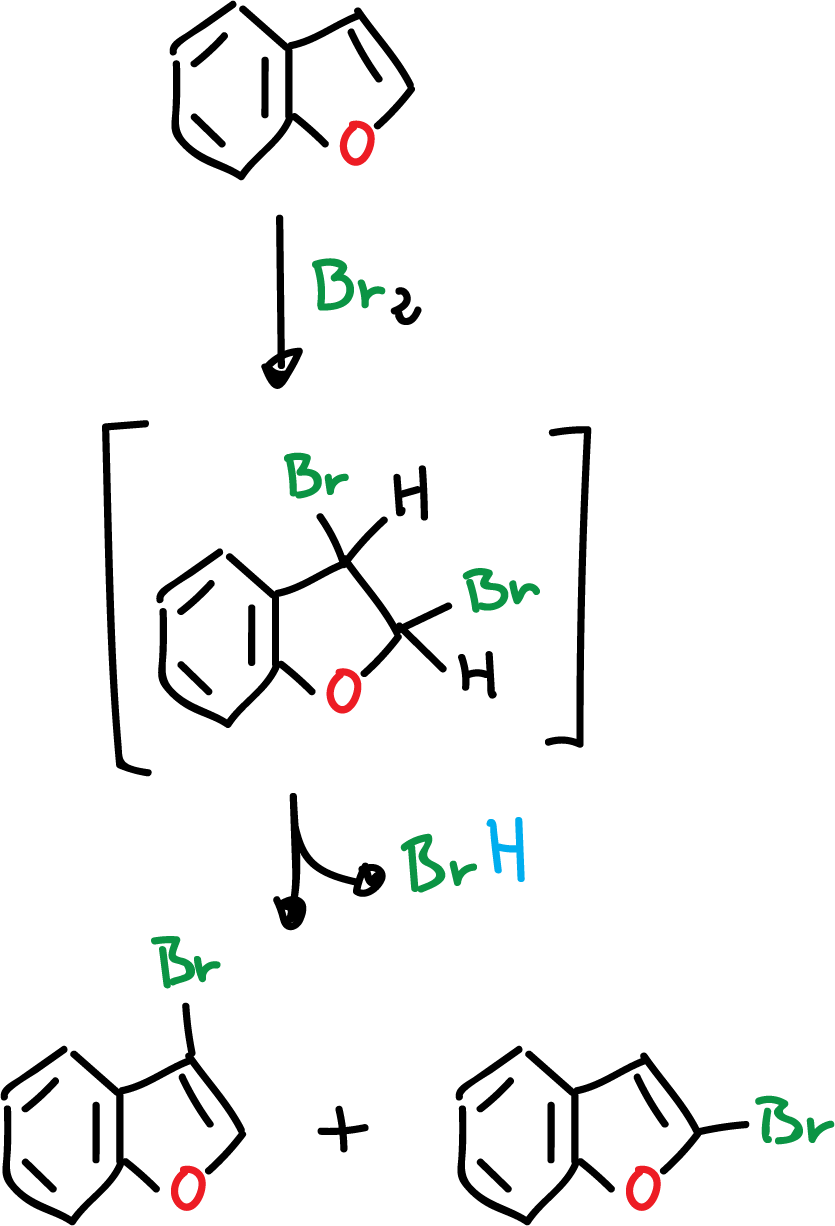
Another reaction that occurs in benzofuran is the direct halogenation with chlorine (Cl2) or bromine (Br2). This halogenation reaction results in addition to the C2=C3 bond, and can even lose hydrogen halide (HCl), to form a mixture of 2- and 3-benzofurans.
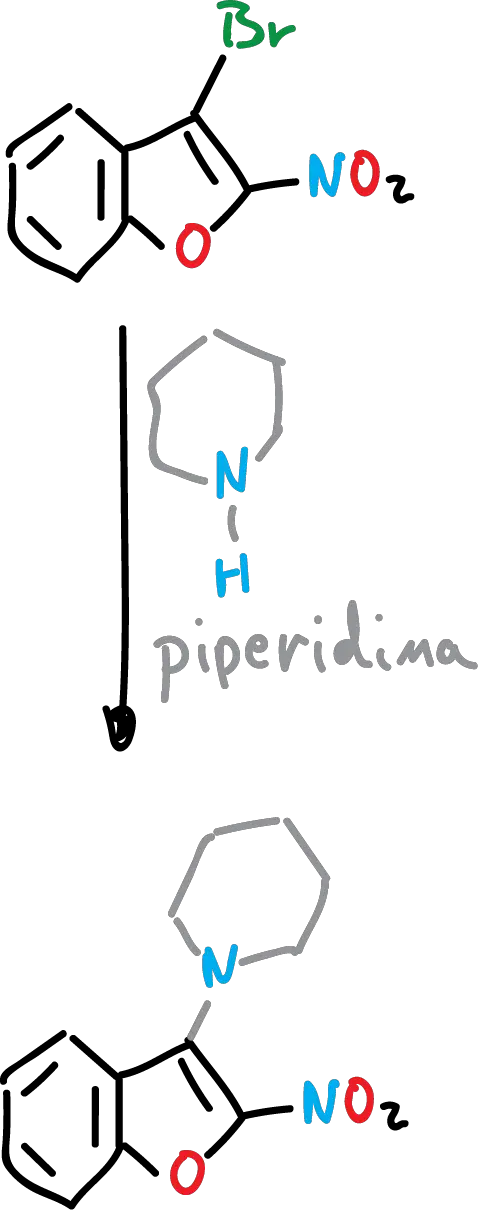
Nucleophilic substitution of benzofurans
These types of reactions have not been extensively studied in benzofurans. However, it is known that, for example, it is difficult for a direct nucleophilic displacement of a halide atom attached to the heterocycle to occur unless the halogen is activated by a neighboring electrophilic substituent.
In addition, it reacts with dichlorocarbenes (Cl2C:), in solution with hexane.
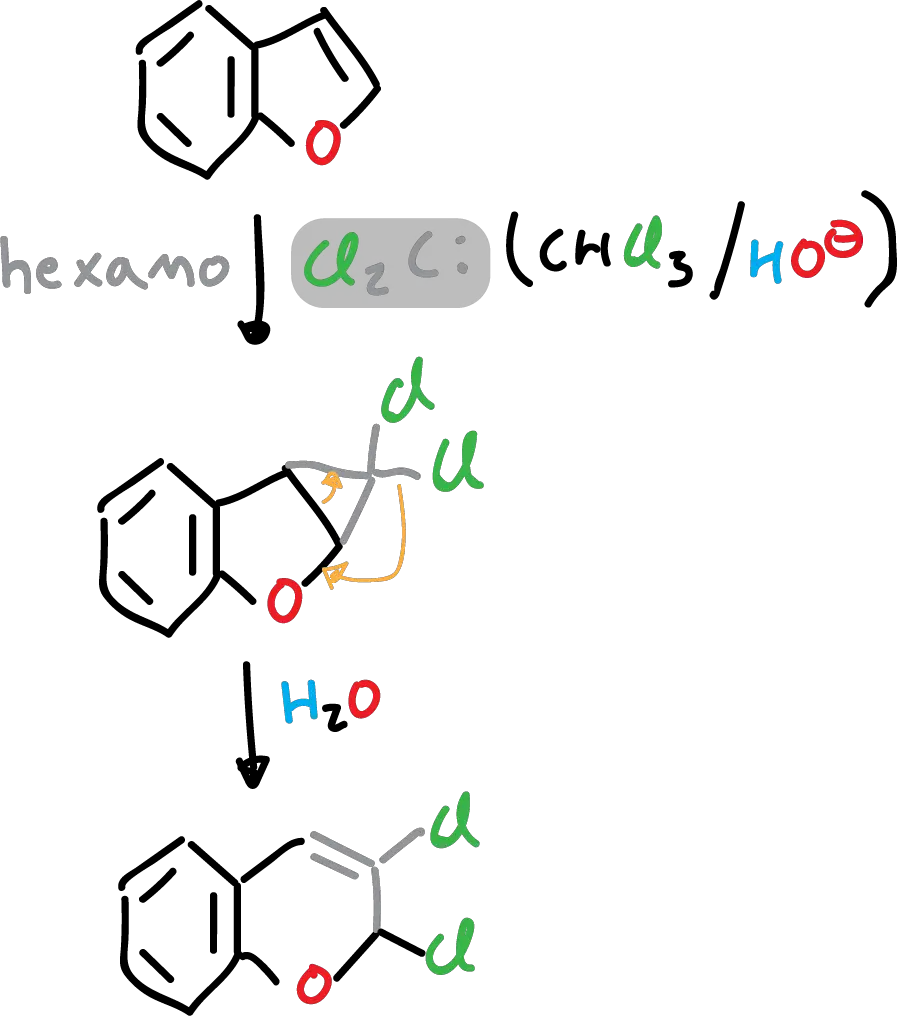
Finally, it should be mentioned that it does not produce Grignard reagent under the usual conditions.
Benzo[b]thiophenes
It occurs in nature, but its most important derivatives are prepared by laboratory synthesis. Its most relevant applications are in the use as colorants, pharmaceuticals, pesticides, etc.
Benzothiophene is a thermally stable solid with a low melting point and a naphthalene-like odor. In nature, it can be found in coal tar.
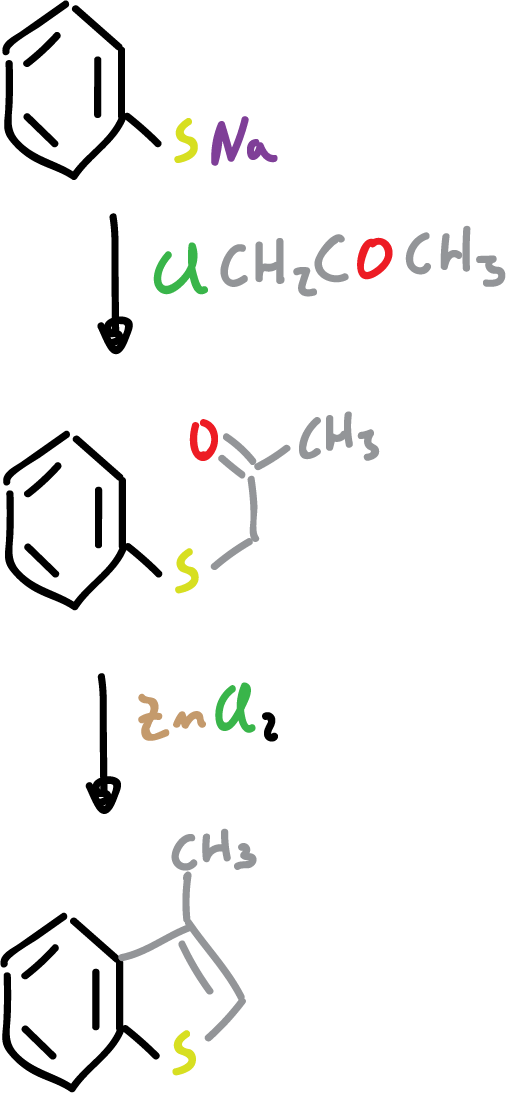
As for their synthesis, the most important methods consist of cyclization reactions using ortho-substituted benzenes as feedstock.
They can also be synthesized from appropriately substituted benzenes by intramolecular Friedel-Crafts-type cyclization.
On the other hand, the reactivity of the five-membered ring system is more stable than in the case of benzofuran.
Electrophilic substitution of benzothiophenes
Such as indole, the C3 position is favored in electrophilic substitutions, although both isomers (at C2 and at C3) are usually formed.
Benzothiophene is less reactive to electrophiles than thiophene.
Some of the most significant examples of electrophilic substitution of benzothiophenes are described below.
Examples of electrophilic substitution of benzthiophenes
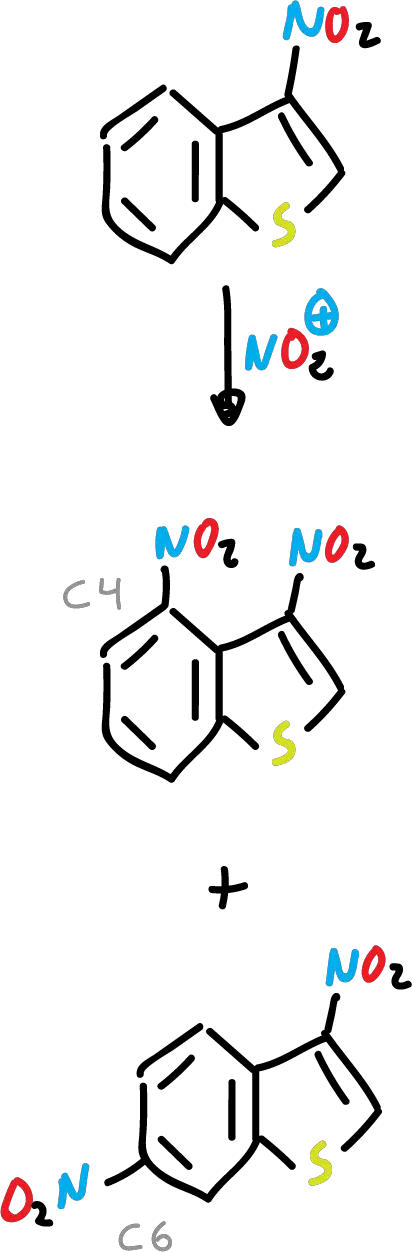
Nitration of benzothiophene with nitric acid (HNO3) and acetic acid (CH3COOH) yields to the corresponding nitro derivative at C3.
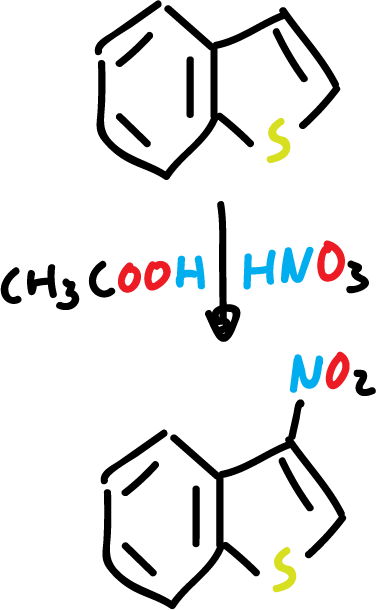
In addition, the presence of an electron-withdrawing substituent at the C3 position deactivates the 5-membered ring, and the reaction is carried out on the benzene ring.
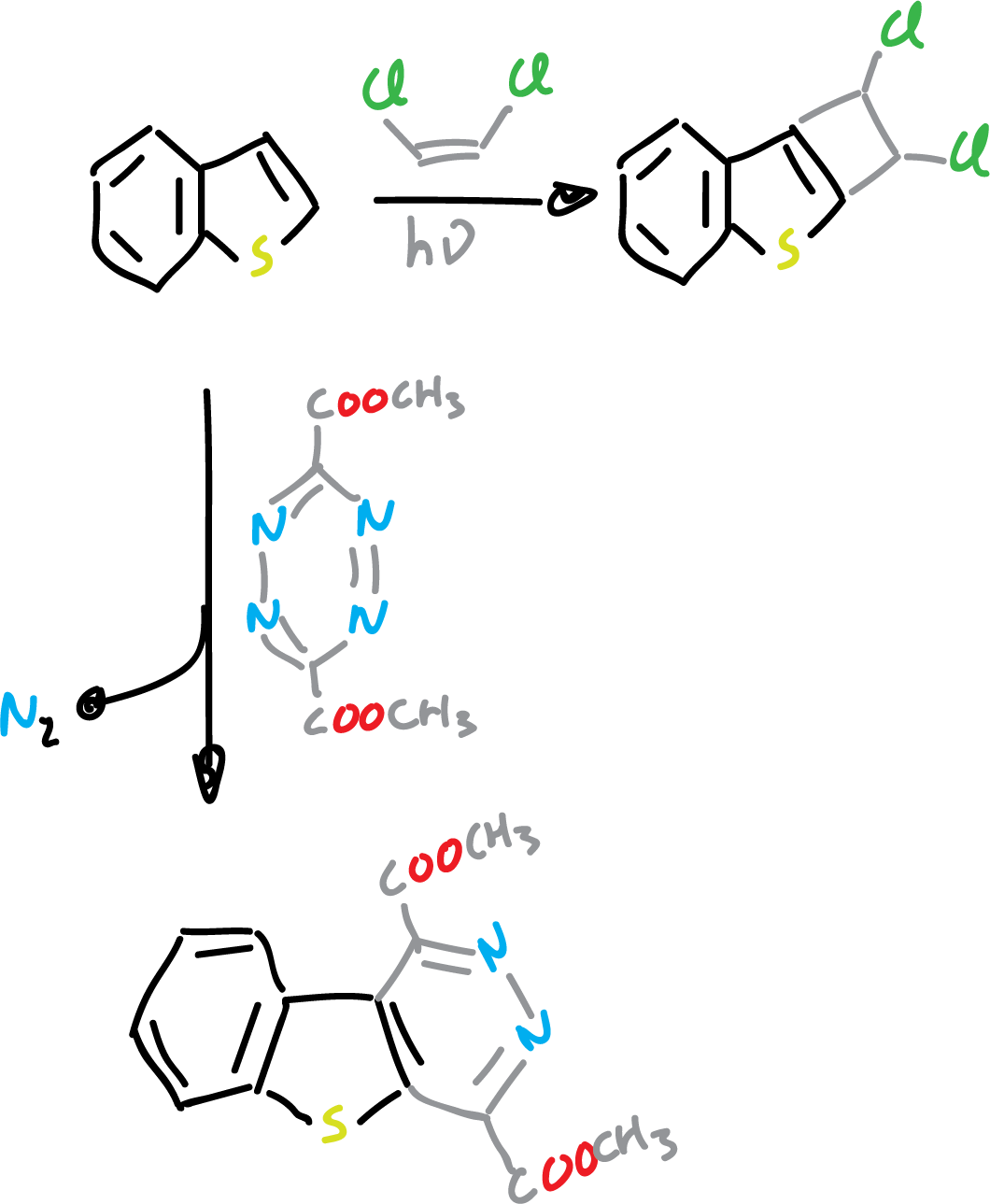
On the other hand, benzothiophene undergoes various types of cycloaddition reactions on the C2=C3 bond.
Benzo[c]fused heterocycles
Heterocycles fused on the c side to benzene are not found in nature. However, they have been synthesized in the laboratory and their properties have been studied.
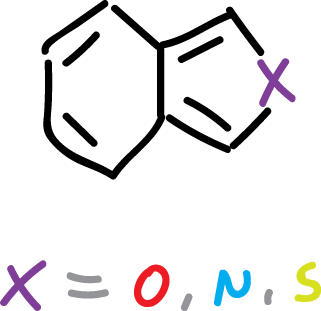
All of them (isoindole, isobenzofuran, benzo[c]thiophene) are highly reactive substances that could only be obtained and characterized at low temperatures. They exhibit lower resonance energies than their b-bonded benzofused analogues, but vary in aromatic character.
The isoindole and benzo[c]thiophene retain an appreciable aromatic character.
Synthesis
Cyclisation reactions have been used to prepare substituted derivatives, but are not suitable for the synthesis of unsubstituted derivatives. 1,2-dibenzoyl benzene is a good starting material for the preparation of derivatives.
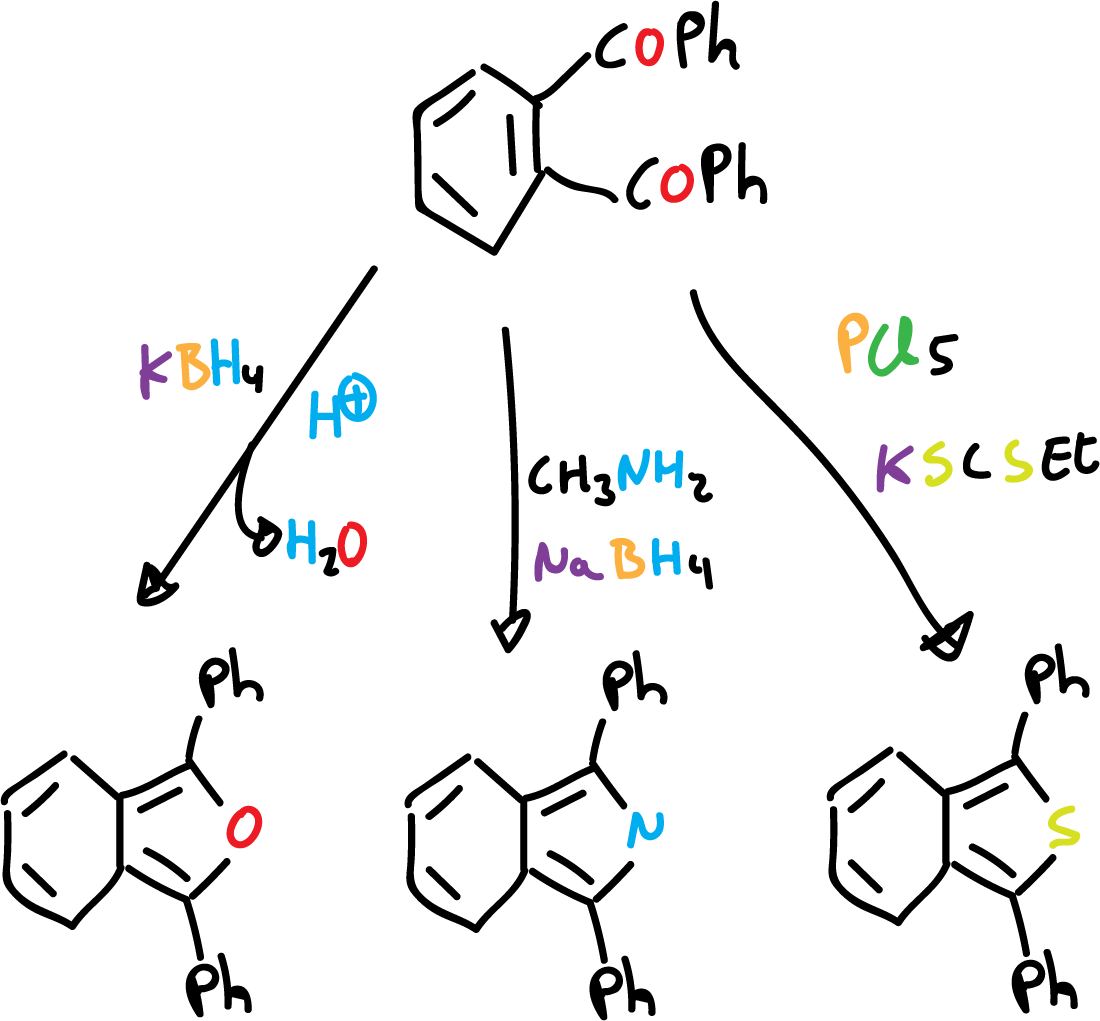
This is analogous to the synthesis of monocycles from 1,4-diketones.
For the preparation of unstable unsubstituted heterocycles it is necessary to use precursors in which the ring systems are already present. Such substituents may be in modified or protected form, so that they do not interfere with the reaction.
For example, benzo[c]thiophene can be prepared by dehydration of S-oxide on alumina.
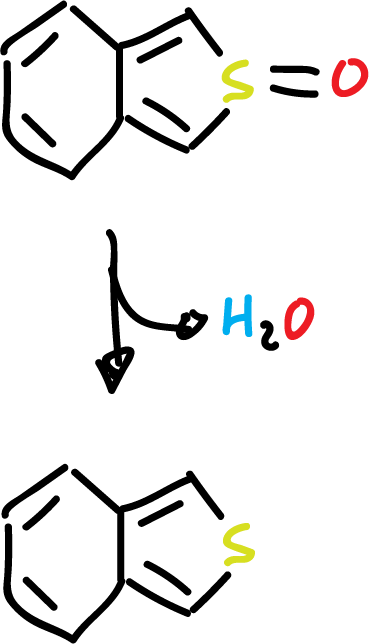
On the other hand, isoindole can be obtained by pyrolysis of the corresponding ester.
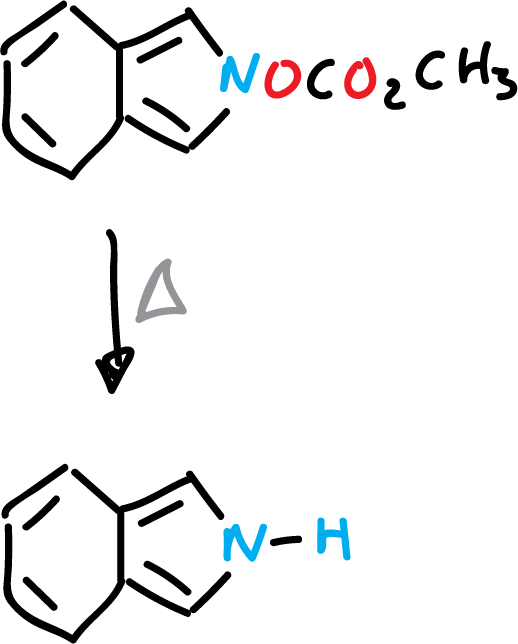
As for their chemical properties, they show characteristics typical of activated versions of their monocyclic analogues. Thus, the C1 and C3 positions (α-carbon atoms of the monocyclic systems) are the most reactive.
Diels-Alder cycloadditions occur very easily in the case of isobenzofuran derivatives.
In addition, isoindoles, which are more aromatic than benzofuran and benzo[c]thiophene, can undergo substitution reactions instead of addition.
Substances related to fused heterocycles
It is relevant to mention in this section other fused heterocycles that are related to the previous ones and are present in nature.
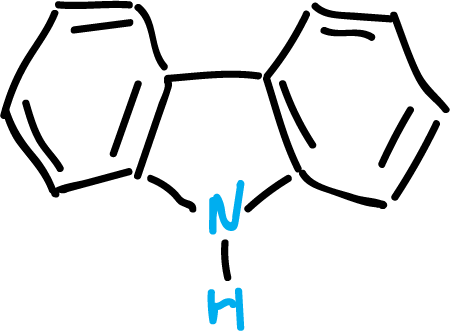
Carbazoles
Carbazole is a heterocycle fused to two benzenes. It is found in coal tar and can also be obtained from some plants as alkaloids.
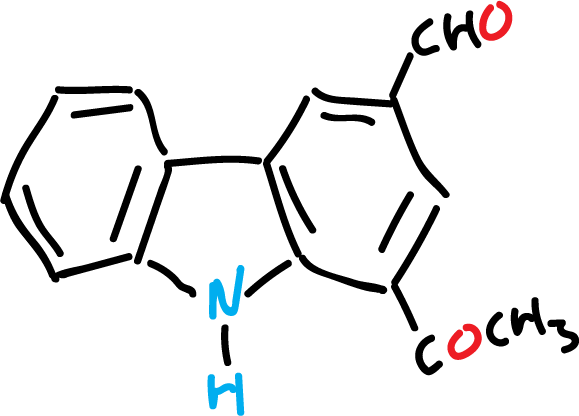
For example, murrayanine, IUPAC name 1-methoxycarboazole-3-carboxaldehyde, and some of its derivatives are used as dyes and for the manufacture of plastics.
Phthalocyanines
Phthalocyanine is a stable blue-green crystalline solid derived from isoindole.
It is prepared by reductive cyclization of 2-cyanobenzamide with magnesium (Mg) or antimony (Sb).
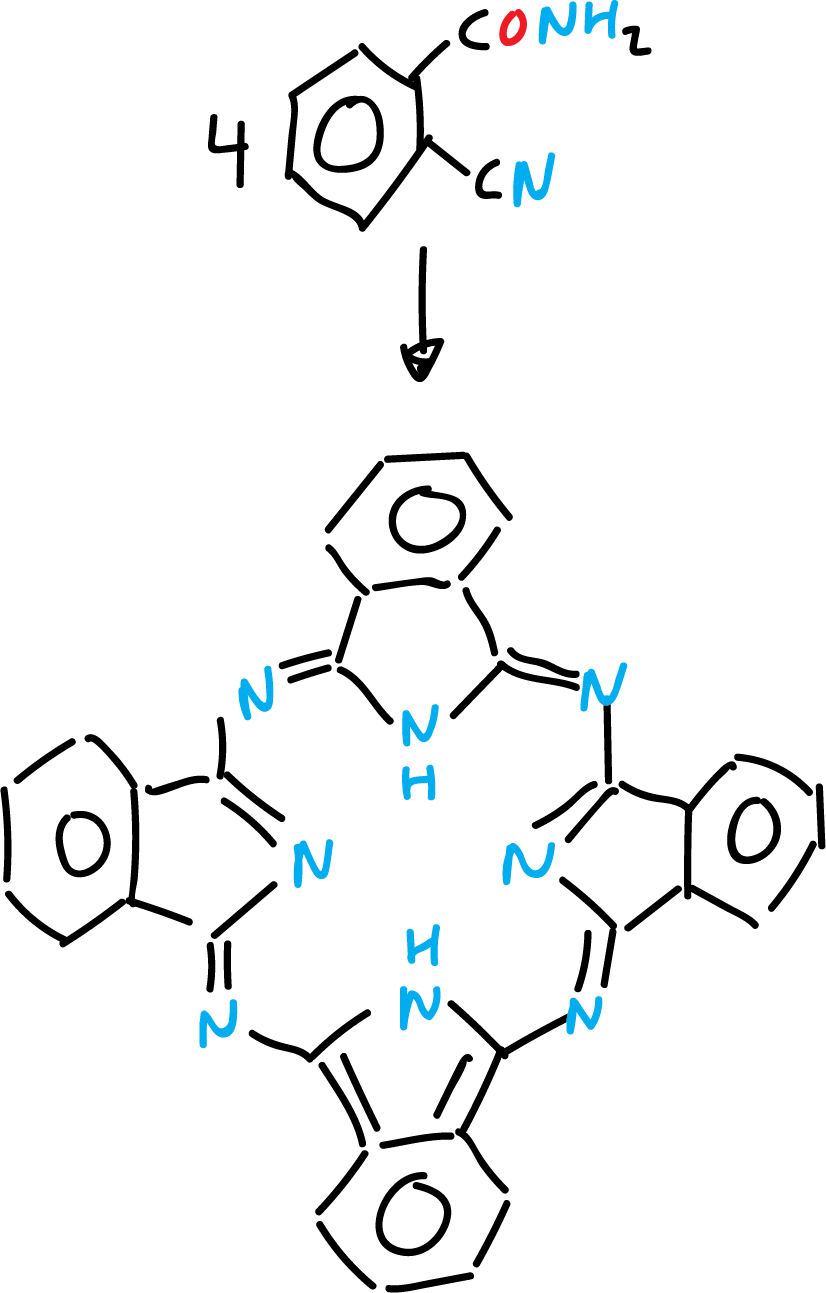
It resembles porphyrins and its complex with copper(II) is an important pigment in the dye industry.