What are cheletropic reactions and group transfer reactions?
They are cycloaddition reactions, however, one of the systems involved is an atom. Normally these reactions are observed in cases of cycloreversion rather than cycloaddition reactions. For example, in this reaction an orbital with a pair of sulfur electrons is involved and so this is actually a cycloaddition which we will name ω2s+π4s (ω denotes that only one orbital of an interacting atom is involved).
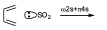
Group transfer reactions are, for example, those in which a saturated system reacts with an unsaturated system and there is a transfer of H from one to the other in such a way that we can say that it is the transfer of a molecule of H2.
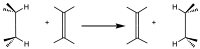
Where if the substituents are equal, logically, it is a degenerate reaction.
Other examples of this type would be:

All these cases are particular cases of cycloaddition reactions. Already the kelotropic reactions are cycloaddition reactions where when we have an σ orbital it has to act suprafacially, but when they are orbitals that can have different signs they can also act antarafacially. All the possibilities can be given the same as in the case of cyclization reactions, what happens is that preferably those that meet the same guidelines that were observed in the cyclization reactions will be given. So for example the above reaction is allowed in the ground state when it is a ω2s+π4s, that is, when there are 6 electrons the supra–supra interaction is allowed. If there were 4 electrons then it would be forbidden.
We are going to see a particular case of a group transfer reaction in which we have a system with a double bond that can interact with a system of this other type in which the interaction can occur as follows:

In this reaction we have on the one hand a π system that intervenes with 2 electrons and does so suprafacially, we have a σ system that intervenes with 2 electrons and also does so suprafacially, another σ system that does so with 2 electrons also suprafacially, and a π system that does so with 2 electrons suprafacially. Therefore, it will be a reaction π2s+σ2s+σ2s+π2s.
So a pericyclic reaction does not necessarily have to have only two electrons but can have many components that are also independent of each other. There the reaction takes place in such a way that the interaction is occurring in the TS in which all the electrons are involved.
In group transfer reactions, the same preferences as mentioned above are also observed. For example, in the ground state that reaction would be forbidden with the sterochemistry we said, since it has 8 electrons. And it is totally similar to the cycloaddition reactions where one of the interactions has to be antara.
With all these definitions that we have seen, it is corroborated that pericyclic reactions follow certain patterns that are always the same. In all of them a TS can be established in which there is a loop of interacting electrons and depending on the nature of that TS the reaction will be allowed or forbidden. The reaction will go through one stereochemistry or another depending on the number of electrons in the TS and its characteristics. Therefore, let’s see if theoretically there are rules that predict whether a reaction is allowed or forbidden and which stereochemistry they will present.