Objetives
This experiment illustrates how to perform chemoselective reductions of 4-nitroacetophenone, a compound with two reducible groups (nitro and carbonyl), which depending on the reducing agents used can reduce one of them without having to protect the other.
Background
Chemoselectivity is the selective reactivity of a functional group in the presence of others. In the absence of chemoselectivity, protecting groups must be used with the drawback of having to perform two additional reactions in the synthesis (protection and deprotection). However, if the reagent and reaction conditions are well chosen, chemoselectivity can be effective.
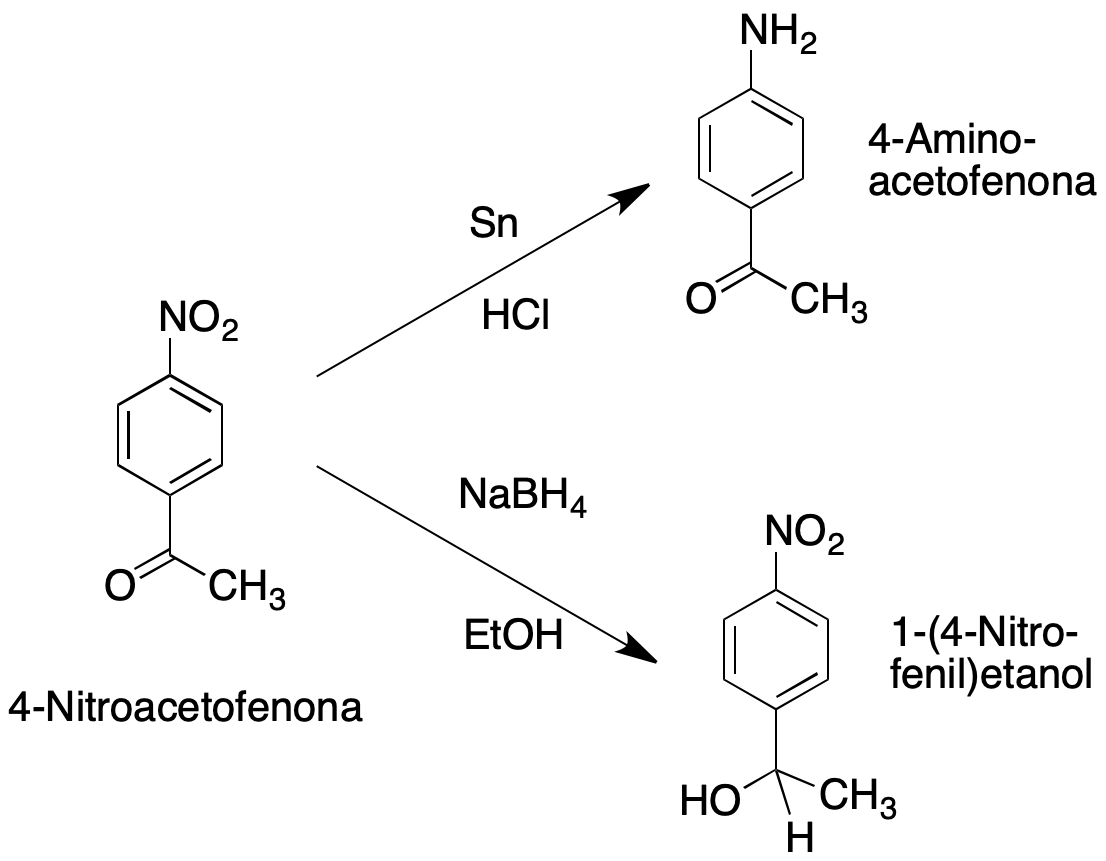
This experiment consists of two parts, and will be carried out on two chemoselective reductions of 4-nitroacetophenone, which is a compound with two reducible groups (nitro and carbonyl).
In the first part, the aromatic nitro group is reduced to aromatic amine using tin and HCl, which does not reduce carbonyl groups.
Alternatively in a second part, the ketone is reduced using the mild hydride transfer agent, NaBH4.
Experimental procedure
A) Reduction of 4-nitroacetophenone using tin and HCl
Cut 3.3 g of tin into small pieces (or use granulated tin) and place them in a 100 ml round bottom flask equipped with a reflux condenser and a magnet. Add 1.65 g of 4-nitroacetophenone and then 24 ml of, water and 9 ml of HCl (conc.). Shake the mixture and heat the flask at reflux for 1.5 h.
Cool the reaction mixture to room temperature. The reaction crude is filtered under vacuum if tin solid remains. Add to the mother liquor slowly ammonia until a precipitate appears, basic pH (NaOH 10% can also be used instead of ammonia). Vaccum filtering and wash with water. Recrystallize in water (melting point 106 °C).
B) Reduction to 1-(4-nitrophenyl)ethanol using NaBH4
Dissolve 1.65 g of 4-nitroacetophenone in 20 ml of hot EtOH in a 100 ml Erlenmeyer flask. Then shake and cool the flask in an ice/water bath if the reaction gets too hot. Add 0.45 g NaBH4 in small portions over 5 min and shake the mixture at room temperature for 15 min.
Add dilute HCl solution dropwise until hydrogen bubbling ceases. Next, add 40 ml of water to the reaction crude, transfer to a 250 ml separating funnel and extract with 2 x 20 ml of CH2Cl2. Combine the organic extracts and dry over Na2SO4. Filter by gravity the drying agent, removing the solvent under reduced pressure (rotovap). The final product, 1-(4-nitrophenyl)ethanol, of the reaction is a liquid.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 1-(4-Nitrophenyl)ethanol | 167.16 | - | - | - |
| 4'-Aminoacetophenone | 135.16 | 103-107 | 293 | - |
| 4'-Nitroacetophenone | 165.15 | 75-78 | 202 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 1-(4-Nitrophenyl)ethanol | See MSDS |
| 4'-Aminoacetophenone |  |
| 4'-Nitroacetophenone | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 1-(4-Nitrophenyl)ethanol | CRJFHXYELTYDSG-UHFFFAOYSA-N |
| 4'-Aminoacetophenone | GPRYKVSEZCQIHD-UHFFFAOYSA-N |
| 4'-Nitroacetophenone | YQYGPGKTNQNXMH-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- A. G. Jones, The selective reduction of meta- (and para-) nitroacetophenone, Journal of Chemical Education 52 (1975), no. 10, 668, DOI: 10.1021/ed052p668