What is Claisen-Schmidt condensation?
The Claisen-Schmidt condensation is a reaction that was first reported by Claisen (University of Bonn) and Schmidt (ETH Zurich) in 1881. It involves the condensation of an aromatic aldehyde with an aliphatic aldehyde or ketone in the presence of a base or an acid to form an α,β-unsaturated aldehyde or ketone with high chemoselectivity. This reaction is sometimes referred to as the Claisen-Schmidt reaction.
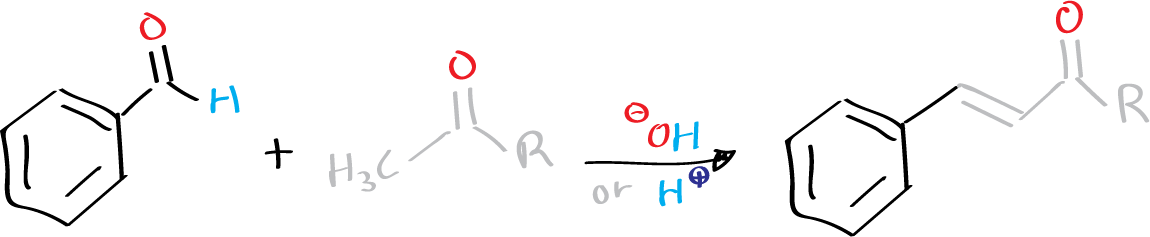
Under basic conditions, the asymmetric ketones react with the aromatic aldehyde through the less substituted position (a methyl group), whereas under acidic catalysis, they react through the more substituted position. However, the reaction is often accompanied by minor side reactions, such as bis-condensation, aliphatic aldehyde dimerization, and Cannizzaro reaction or Tishchenko reaction of aromatic aldehydes.
It has been observed that the reaction between arylaldehydes and cycloalkanones predominantly affords α,α-diarylidenecycloalkanones instead of α-arylidenecycloalkanones, and the generated exocyclic bonds are exclusively trans with respect to the aryl ring and the carbonyl group of the cycloalkanone.
The Claisen-Schmidt condensation has been applied to the preparation of chalcone, flavanone, 1,3-diarylpropane derivatives, and a new family of macrocycles in a single step.
Microwave activation in a polar solvent or without solvent has been shown to accelerate the reaction and enhance the yield while reducing the reaction time.
Additionally, magnesium oxide MgO crystal has been used as a catalyst, which can be reused up to five times without loss of activity and selectivity. However, it has been observed that the Claisen-Schmidt condensation of benzaldehydes and acetopheones with substituent groups in either of the two aromatic rings occurs at a slower rate than the unsubstituted reactants.
References
- Schmidt, J.G. (1881), Ueber die Einwirkung von Aceton auf Furfurol und auf Bittermandelöl bei Gegenwart von Alkalilauge. [On the effect of acetone on furfurol and on bitter almond oil in the presence of alkali metal hydroxide solution] Ber. Dtsch. Chem. Ges., 14: 1459-1461. https://doi.org/10.1002/cber.188101401306
- Claisen, L. and Claparède, A. (1881), Condensationen von Ketonen mit Aldehyden. [Condensations of ketones with aldehydes] Ber. Dtsch. Chem. Ges., 14: 2460-2468. https://doi.org/10.1002/cber.188101402192
Articles on the website