What is Column chromatography (CC)?
Column chromatography (CC) is a method frequently used for the purification of substances and the separation of mixtures or on a preparative scale. It uses as its main device, a chromatography column. The column is packed with an adsorbent that acts as a stationary phase, usually silica gel or alumina.
The sample is introduced into the column and the running solvent or mixture of solvents (eluent) causes the separation of impurities from the desired product or the separation of the components of a mixture, due to the different retention time in the stationary phase. Running the eluent through the column can be done by gravity or by exerting pressure with an inert gas or air by means of a pump.
Flash Chromatography
When the aid of pressure is used to drive the eluent through it is referred to as “flash chromatography“. In this case, the column must have a ground glass joint[1] at the top to which is connected an adapter with an air exhaust connector to which is attached a flexible tube through which the gas is supplied under pressure.
Flash chromatography is very efficient and has the advantage of being much faster than gravity chromatography. The stationary phase used in this technique is specific for this technique, since it has a smaller particle size than conventional chromatography.
This is because finer sorbent particles mean a longer effective path for the eluent, which is beneficial the higher the flow rate of the mobile phase, as is the case in flash chromatography.
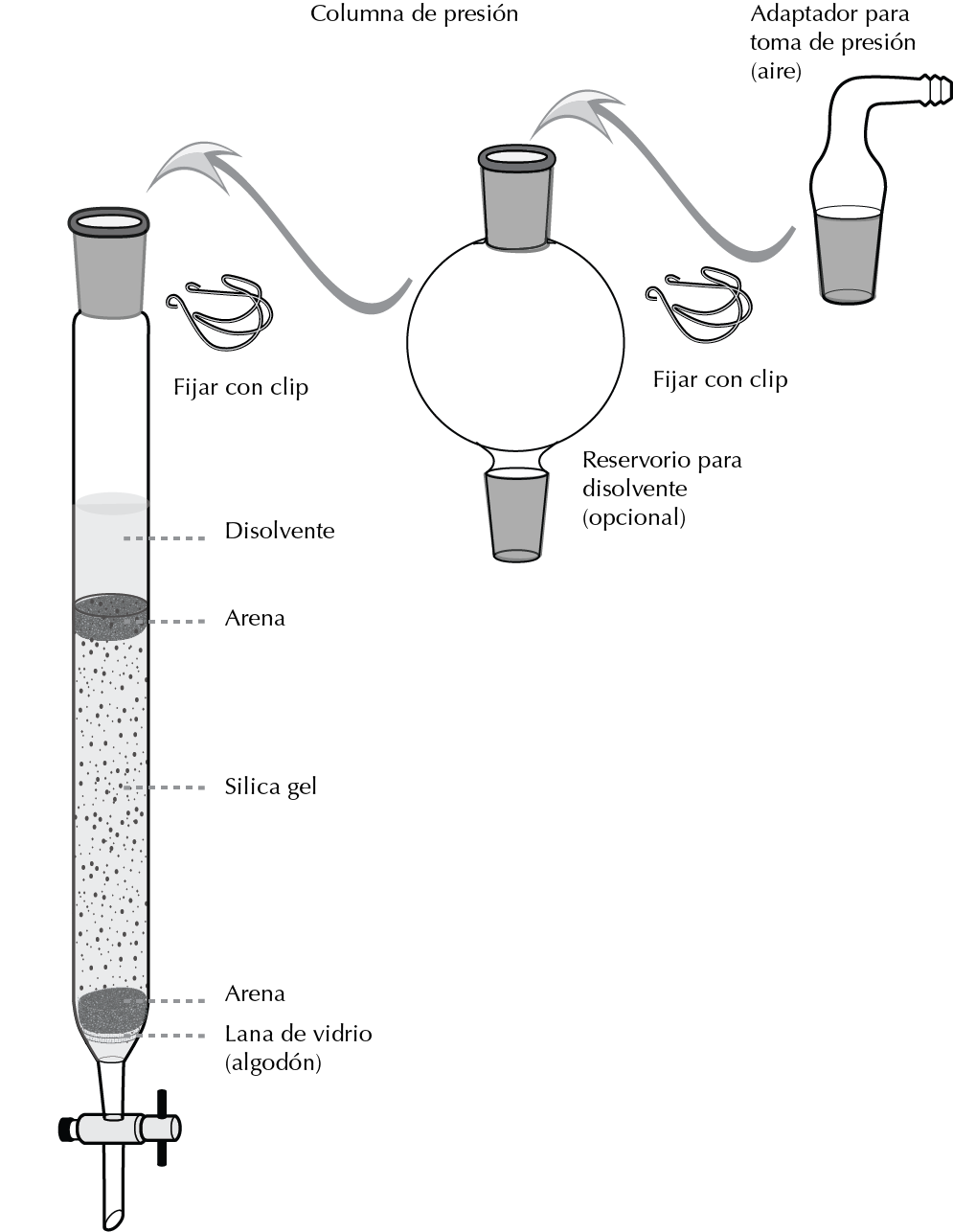
Procedure to perform a flash CC with silica gel.
Choice of mobile phase (eluent)
The choice of solvent is crucial for good separation. If the solvent to be used is very polar, the elution will be fast and there will be little separation between the product and the impurities or between the components of the mixture.
If, on the other hand, the solvent is very non-polar, the compounds will be retained on the column. Therefore, several TLC plates with silica gel should be developed to determine which solvent or combination of solvents is the most suitable so that the spots observed are as far apart as possible.
Stationary phase
The amount of silica gel to be used depends on the amount of sample to be purified and the Rf of the stains on the TLC.
As a guideline, for a sample with a pair of spots, Rf around 0.5 and TLC showing appreciable separation between them, the amount of silica gel (of the 40-75 μm type) per unit mass of sample would be approximately 50:1.
The column is fixed with a clamp to a support and the stationary phase is introduced forming a slurry with the same eluent that is going to be used later with the help of a solid funnel. If the column has a porous glass plate at the base it can be added directly, otherwise a cotton ball is placed with the help of a compacted glass rod so that it retains the silica gel in the column and lets the eluent pass through.
Therefore, it is important that the frosted setting of the column is not fouled by the silica gel, so any debris should be washed away with solvent. A little sand can be placed at the boundary of the silica gel to prevent it from being stirred when the eluent is added. The column size should be large enough to leave a free volume to allow the eluent to be added.
Sample application
Generally, the sample is introduced into the column by adjusting the eluent level just at the limit of the silica gel. If the sample is soluble in the eluent, the eluent is dissolved in the smallest possible amount and transferred to the column with a dropper, over the silica gel, avoiding staining the column walls.
In case it is insoluble, a solution of the sample is prepared in a round bottom flask to which silica gel is added. The solvent is evaporated under reduced pressure at a rotary evaporator with a foam breaker between the guide tube and the flask to prevent the silica gel from jumping out of the flask into the guide tube.
An analogous effect to the foam breaker can be obtained if a spatula is introduced into the flask, taking care that it does not impede the rotation of the flask.
Once the solvent has evaporated, the silica gel with the adsorbed product on its surface is introduced into the column.
Addition of eluent
Once the sample has been introduced into the holder, the eluent is added, an adapter with a side outlet connected to the pressure equipment or pump with a flexible tube is placed. It is secured with a clip and pressure is applied. An additional reservoir for the solvent can be used by sandwiching it between the column and the pressure outlet.
Elution rate
For each chromatographic separation there is an optimum elution velocity value. If the rate at which the eluent flows is too slow, the sample diffuses excessively into the column, the separation run time is lengthened and the separation efficiency decreases.
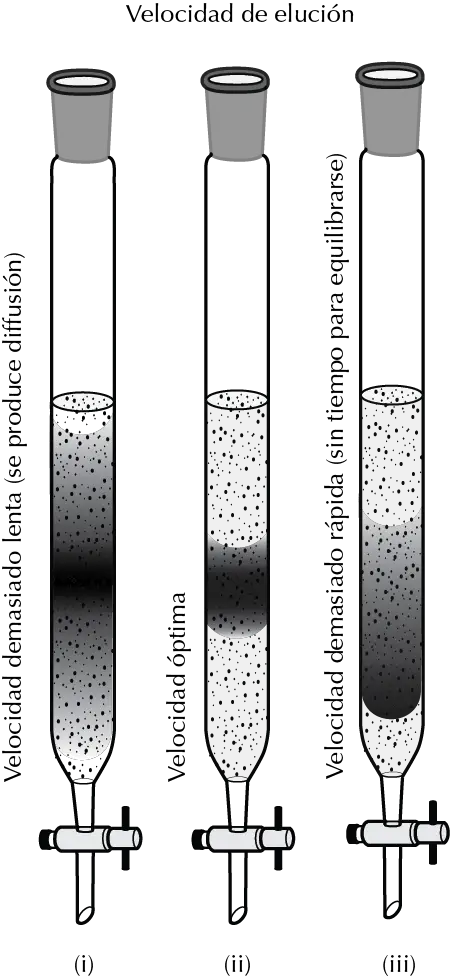
If on the other hand the eluent flow is too fast, there is no time for the sample to interact with the column packing and the separation is not efficient.
On the other hand, to achieve efficient separation in small diameter columns, the eluent flow must be much slower than in larger ones.
Eluent collection and product isolation
The eluent is usually collected in test tubes, which should be placed in an orderly manner in a rack. In order to know in which fractions the product or products that have been purified are present, an analysis of these fractions is made by TLC.
Once it is known in which fractions the products are found, the corresponding fractions are collected in a round bottom flask and the solvent is removed (rotary evaporator).
References and notes
- [1] Do not forget to fix the ground joint with a Keck clamp or metal clip.
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2