What are cycloaddition reactions?
In cycloaddition reactions, as the name implies, a cyclic system is generated from the various components. They are usually named with numbers indicating the π electrons of each component. An example would be the two ethylene molecules to form cyclobutane:
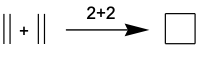
This reaction would be a case of a 2+2 reaction, since one of the components has two electrons and the other also has two electrons.
Another example is ethylene plus butadiene to give cyclohexene, this would be a 2+4 reaction.
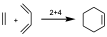
Historically, in pericyclic reactions the transition state (TS) is usually represented by a notation with arrows indicating the movement of electron pairs, so that for the above examples we will have:
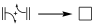

These arrows indicate the movement of electron pairs from the reactants to move to the TS, and subsequently to the product of the reaction. Whether that movement is to the right or left is completely arbitrary and only gives us an idea of how the electron pairs can be redistributed, but the only true thing is that in the TS the π electron pairs involved in the reaction form a closed loop.
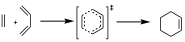
Thermal and photochemical reactions
Cycloaddition reactions (there are also 4+4, etc.) have some very interesting characteristics. The first is that experimentally it is observed that 2+2 or 4+4 reactions hardly occur in the fundamental state, i.e. thermally. Whereas the Diels-Alder type reactions (which are 2+4) occur very easily. But if we test these reactions with some of the reactants in their excited state (photochemical reactions), the opposite happens, i.e., now the 2+2 or 4+4 reactions occur easily while the Diels-Alder reaction in the excited state is very difficult to observe.
Within the cycloaddition reactions it is worth highlighting the existence of some reactions that are widely used in heterocycle synthesis since they occur very easily, and they are the 1,3-dipolar cycloaddition reactions which is the reaction between a 2-electron π system with a 1,3-dipole which is a system with 4 electrons and three centers (which can usually be represented by different resonant forms). These reactions are 2+4, to give rise to 5-membered cyclic systems.
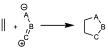
They usually occur at low temperature and are easily observed. Among the best known dipoles used in 1,3-dipolar reactions are O3, NO2, CON (nitrile oxide), COO (carbonyl oxide).
Thus, in the case of ozonolysis, O3 reacts very easily with olefinic systems, where it is postulated that this is the first product of the reaction.
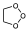
But which is a very unstable compound that breaks down in a reverse cycloaddition reaction but instead of generating the starting products, it breaks down in such a way as to give, on the one hand, a carbonyl group and, on the other hand, carbonyl oxide.
Also with NO2 or CN groups, different types of heterocycles can be accessed.
Stereochemistry of cycloaddition reactions
Cycloaddition reactions in general have to react with a certain stereochemistry that can be observed for example in the Diels-Alder reaction, where we are going to have the π system of cyclobutane in the simplest way we can imagine ethylene can react, this interaction of the π systems would be as indicated:
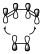
Where the π system of 2 carbon atoms interacts with a stereochemistry in such a way that if we have the flat butadiene, the π system of two electrons interacts underneath in such a way that the lobes of the orbitals interact. This interaction is performed on the same side of a 2-electron π-system and on the same on the other 4-electron π-system. When one of the components of the cycloaddition reaction, or one of the π-systems, intervenes in the reaction with both ends of its π-system on the same side, it is said to proceed in a supra-facial manner. Whereas if a π-system does so with both ends but with different faces, it is said to do so antarafacially.
So if we have our system with different substituents:
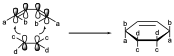
The interaction of that π system of 2 electrons is with both ends on the same side and is represented by first indicating whether the electrons involved in the reaction are π or s, in this case both are of π type, then we indicate the number of electrons, in this case 2, and finally the stereochemistry, in the suprafacial example, so the system would be π2s. Since we have another π system with 4 electrons the Diels-Alder reaction will beπ2s+π4s, a reaction involving both π systems in a suprafacial manner, and this has importance, since the stereochemistry of the interaction implies stereochemistry in the products, since in our case it will cause the b substituents to go up and the to substituents to go down. This system going from sp2 to sp3 hybridization will cause the two b-substituents to go up and the two a-substituents to stay equatorial. The same in the case of the 2-electron system, the c substituents will be on the same side and the d substituents will be on the same side. So if we had a certain stereochemistry, it is still conserved, if the 2-electron π system had a cis configuration, the configuration of the reaction products is still cis.
Logically, we can also have the products in which the two substituents b were downward. But the interesting thing is that the stereochemistry of the original 2-electron π system is maintained in the reaction product, i.e., the reaction is fully stereospecific.
A reaction in which one of the components acts in an antarfacial manner will involve a different stereochemistry. For one of the systems to act antarfacially, it has to intervene on both sides of the π-system. In a two-electron system it will be quite difficult for it to intervene from above and below, since this would imply a very large deformation, therefore we will have to consider that the 2-electron system is going to approach with a totally different orientation if we want one of the components to act in an antarfacial way, and that different orientation for one of them to intervene in an antarfacial way must be:

It is an orientation in which the 2-electron system is in the plane of the paper, and the 4-electron system is in a perpendicular plane, such that one of the lobes can interact with the top of the 4-electron system, while the other can interact with the bottom.
This orientation implies a stereochemical change in the substituents of those systems, so that would result in a product where the original part of the two-electron system maintains the stereochemistry, however the stereochemistry of the carbons that were part of the 4-membered system has to change.
So if the reaction is supra-supra it implies retention of the configuration of the different chiral centers involved in the reaction. If the reaction is supra-antara it implies the change of configuration of one of the chiral carbons formed.
In addition, as a characteristic we had also seen that whether a reaction occurs or not, has to do with the number of electrons involved in the reaction. Thus we have seen that reactions with 4 electrons do not occur easily while those with 6 electrons do occur. But this ease of reaction is associated with stereochemistry. That is, the Diels-Alder reaction occurs very easily when both components act supra-facially. All the examples of Diels-Alder reactions in the ground state that have been studied involve stereospecificity and in addition the stereochemistry of the reactants in the products is conserved.
That is, if a pericyclic reaction (not only cycloaddition) occurs very easily and we add 2 electrons to the system involved in the reaction now it becomes very difficult to occur. If we have a pericyclic reaction with a certain number of electrons and a stereochemistry and it happens easily, if we change to another reaction with the same number of electrons but in such a way that we change the stereochemistry of one of the components, the reaction becomes difficult to happen. And this is a general thing for all the pericyclic reactions that we will see. Moreover, it will allow us to extract theoretical rules to predict whether or not and with what stereochemistry, depending on the number of electrons in the reaction.
Logically, everything we have said with respect to the ease and stereochemistry of pericyclic cycloaddition reactions is reversed when we move from thermal to photochemical reactions. That is, if one occurs easily with a certain stereochemistry in the ground state, it will be very difficult to occur with that stereochemistry (but it can occur with the stereochemistry changed) when passing to the excited state.
From these examples we have seen it can be summarized that the most normal is that supra-supra reactions occur, but we will see that the other possibilities can also occur depending on the reaction conditions and the number of electrons. Although if we have a cycloaddition reaction and it is a permitted supra-supra reaction, then the supra-antara will be forbidden, while the antara-antara will again be permitted.