Objective
The objective of this practice is the realization of a Diels-Alder cycloaddition between a diene and an alkene to obtain a highly functionalized cyclohexene derivative.
Background
Diels-Alder reaction is one of the most remarkable chemical reactions discovered in the 20th century. The authors of this reaction won the Nobel Prize in 1950. It is a powerful tool for constructing 6-membered rings and has been used for the synthesis of many complex molecules.
![Diels-Alder reaction: a) generation of buta-1,3-diene from 3-sulfolene and reaction with maleic anhydride. b) [4+2] cycloaddition between (2E,4E)-hexa-2,4-dien-1-ol and maleic anhydride](https://www.chemistry-online.com/wp-content/uploads/2022/11/sch-DA-butadiene.png)
Experimental procedure
[4+2] Cycloaddition between maleic anhydride and buta-1,3-diene
In a 100 ml round flask 10 g of 3-sulfolene (buta-1,3-diene sulfone) and 5 g of maleic anhydride powder are introduced. Subsequently, 10 ml of xylene are added and the flask is refluxed with magnetic stirring for 45 min. Since SO2 is released during the reaction, a gas trap containing a 5 % NaOH solution is placed at the end of the condenser.
![[4+2] Cycloaddition between maleic anhydride and buta-1,3-diene from 3-sulfolene](https://www.chemistry-online.com/wp-content/uploads/2022/11/sch-DA-butadiene-step1.png)
Cool the solution to room temperature and add 50 ml of toluene and activated carbon to decolorize the solution. Filter by gravity. To the filtrate add 25-30 ml of hexane, heat to 80 ºC. Cool the solution first to room temperature and then in an ice bath. Filter the formed crystals under vacuum.
[4+2] Cycloaddition between maleic anhydride and E,E-2,4-hexadien-1-ol
A mixture of 0.40 g maleic anhydride and 0.40 g E,E-2,4-hexadien-1-ol in 5 ml toluene is heated to reflux for 5 min. The solution is allowed to cool slowly to room temperature, whereupon a solid will begin to appear on the walls of the flask.
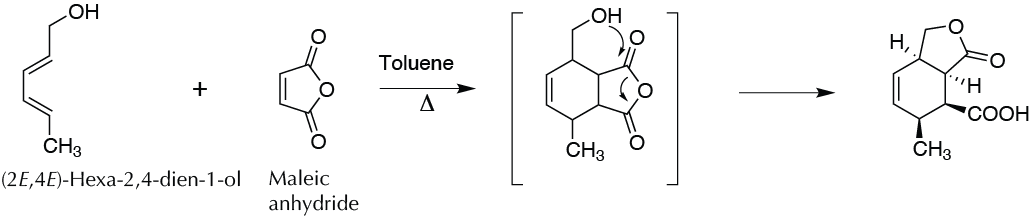
To assure the total precipitation of the product, (3aR,4S,5S,7aR)-5-methyl-3-oxo-1,3,3a,4,5,7a-hexahydro-2-benzofuran-4-carboxylic acid, we cool in an ice bath for 10 min. It is filtered under vacuum and dried in the air. It can be recrystallized from toluene. (melting point 156-159 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Sodium sulfite | 126.04 | - | - | 2.630 |
| Buta-1,3-diene | 54.09 | -109 | 4.5 | 0.62 |
| p-Xylene | 106.17 | 13.0 | 138.4 | 0.860 |
| 3-Sulfolene | 118.15 | 64-67 | - | - |
| cis-1,2,3,6-Tetrahydrophthalic anhydride | 150.147 | 97-103 | - | - |
| 2,4-hexadien-1-ol | 98.14 | 27-33 | 80 | 0.871 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Maleic anhydride | 98.06 | 51-56 | 200 | 1.480 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| SO2 | 64.06 | -73 | -10 | - |
| Toluene | 92.14 | -93 | 110.6 | 0.867 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Sodium sulfite | Non-hazardous |
| Buta-1,3-diene |    |
| p-Xylene |   |
| 3-Sulfolene |  |
| cis-1,2,3,6-Tetrahydrophthalic anhydride |   |
| 2,4-hexadien-1-ol |   |
| HCl |   |
| Maleic anhydride |    |
| Hexane |     |
| NaOH |  |
| SO2 |    |
| Toluene |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Sodium sulfite | GEHJYWRUCIMESM-UHFFFAOYSA-L |
| Buta-1,3-diene | KAKZBPTYRLMSJV-UHFFFAOYSA-N |
| p-Xylene | URLKBWYHVLBVBO-UHFFFAOYSA-N |
| 3-Sulfolene | MBDNRNMVTZADMQ-UHFFFAOYSA-N |
| cis-1,2,3,6-Tetrahydrophthalic anhydride | KMOUUZVZFBCRAM-OLQVQODUSA-N |
| 2,4-hexadien-1-ol | MEIRRNXMZYDVDW-MQQKCMAXSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Maleic anhydride | FPYJFEHAWHCUMM-UHFFFAOYSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| SO2 | RAHZWNYVWXNFOC-UHFFFAOYSA-N |
| Toluene | YXFVVABEGXRONW-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- T. E. Sample and L. F. Hatch, 3-Sulfolene: a butadiene source for a Diels-Alder synthesis: an undergraduate laboratory experiment, Journal of Chemical Education 45 (1968), no. 1, 55, DOI: 10.1021/ed045p55