What is dopamine?
dopamine (molecular formula C8H11NO2) is an organic molecule with neuromodulatory properties and an ancestor of epinephrine (adrenaline) and norepinephrine (noradrenaline). It is a key transmitter in the brain’s extrapyramidal system and is crucial in the synchronisation of movement. A group of receptors (dopamine receptors) facilitates its function.
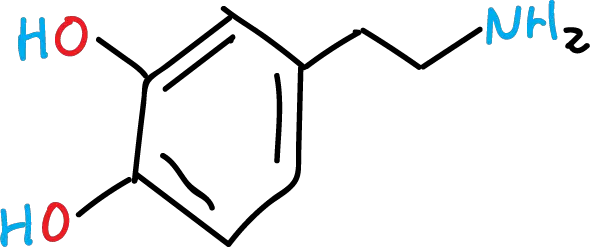
Its IUPAC name is 4-(2-aminoethyl)benzene-1,2-diol.
 |
| 3D Structure |
Dopamine has a catechol chemical structure (a benzene ring with two OH, hydroxyl groups) with an ethyl substituent terminating in an amino group (NH2).
Physico-chemical properties
Dopamine has a pKa of 8.93. Its melting point is 128 ºC and its boiling point is 227 ºC. Its molar mass is 153.18 g/mol and it has a water solubility of 60.0 g/100 mL.
Because it has an amino group, dopamine is a base and is protonated in acidic media. The protonated form is very soluble in water and stable, but oxidizes if exposed to air (oxygen) or other oxidants. In basic media it is not protonated and this free base form is less soluble in water and more reactive.
Because of the greater stability and water solubility of the protonated form, dopamine is supplied for chemical or pharmaceutical use as dopamine hydrochloride, i.e., the hydrochloride salt that is created when dopamine is combined with hydrochloric acid.
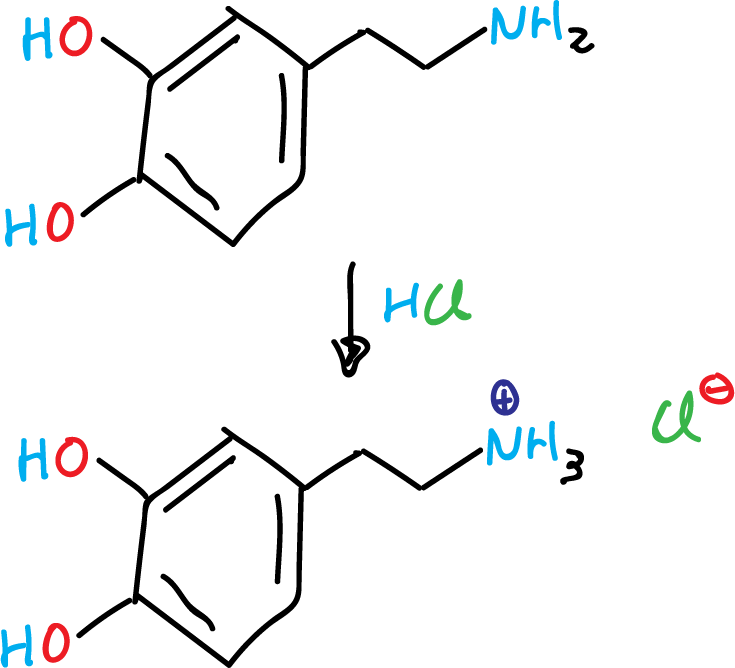
Dopamine hydrochloride is a fine white to yellow powder.
Dopamine biosynthesis
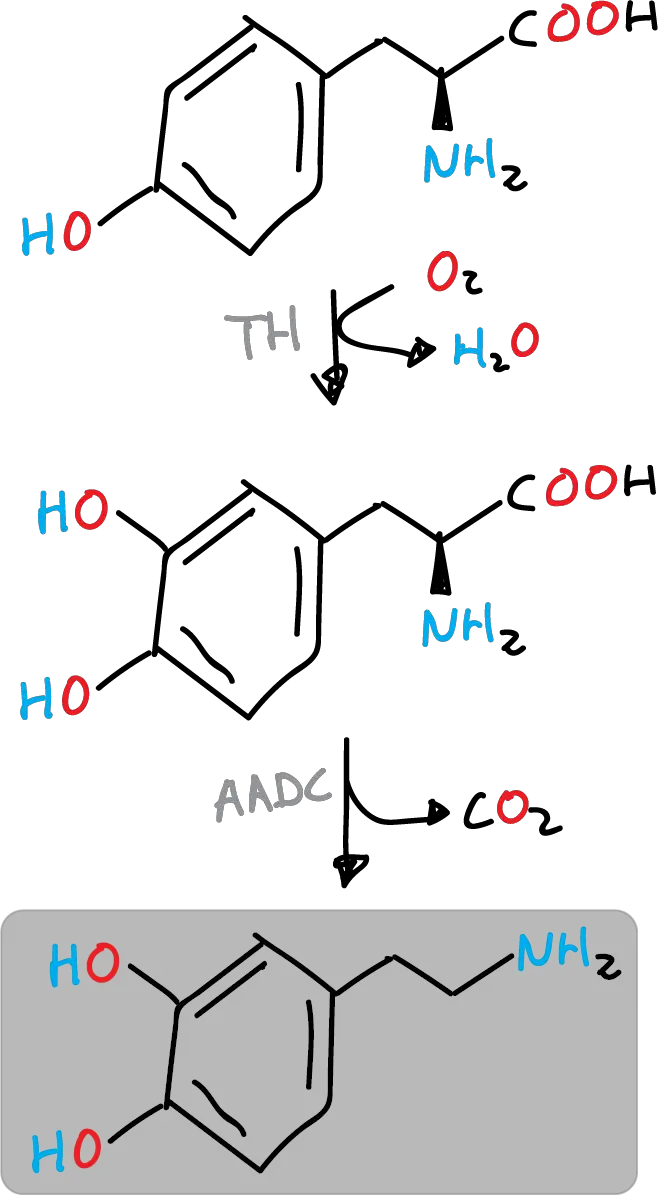
Dopamine is produced in the body (nervous tissue and adrenal glands) initially by the hydration of the amino acid tyrosine to DOPA by tyrosine hydroxylase (TH) and subsequently by the decarboxylation of DOPA by the enzyme aromatic L-amino acid decarboxylase (AADC), also called DOPA decarboxylase (DDC). TH is the rate-limiting enzyme in the biosynthesis of dopamine and other catecholamines.
Dopamine as a drug
The brain’s reward system is strongly associated with feel-good dopamine. In certain areas of the brain, such as the nucleus accumbens and the prefrontal cortex, dopamine release is mainly due to satisfying experiences (food, drugs, physical exercise, sex, learning new tasks, understanding unfamiliar things and the neural stimuli correlated with these actions).
Parkinson’s disease is a degenerative disorder that causes tremors and motor disturbances and is instigated by the loss of dopamine-secreting neurons in an area of the midbrain called the substantia nigra.
Dopamine agonists, by mimicking dopamine, bind to and activate dopamine receptors, mirroring dopamine activity, thus tricking the brain into thinking it has dopamine, giving it the biological functions of dopamine. Hence, these agonists are used as therapeutic agents in the treatment of depression and Parkinson’s disease. The replenishment of dopamine levels through L-DOPA improves the symptoms of Parkinson’s disease.
Schizophrenia involves distorted levels of dopaminergic activity, and most of the antipsychotic drugs used to cure it are dopamine antagonists, which reduce dopaminergic activity. Therefore, antagonists have an important therapeutic value in the treatment of psychiatric conditions such as schizophrenia, which are the result of an overexcited dopaminergic organization.
In addition, attention deficit hyperactivity disorder, bipolar disorder and addiction are also characterized by defects in dopamine production or metabolism.
However, when present at sufficiently high levels, dopamine can be a neurotoxin (chemical that alters neural tissue) and a metabotoxin (endogenously produced metabolite that causes adverse health consequences at persistently elevated levels).
Thus, chronically elevated dopamine levels are associated with neuroblastoma, Costello syndrome, leukemia, pheochromocytoma, decarboxylated aromatic L-amino acid deficiency and Menkes disease. In addition, these high levels of dopamine can cause hyperactivity, insomnia, anxiety, depression, delirium, excessive salivation, nausea and digestive problems.
International Chemical Identifier
We provide identifiers InChI key of the IUPAC for, the main compounds described in this section in order to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| dopamine | VYFYYTLLBUKUHU-UHFFFAOYSA-N |
| dopamine hydrochloride | CTENFNNZBMHDDG-UHFFFAOYSA-N |