What is E621?
E621, or sodium glutamate or monosodium glutamate (MSG), is the sodium salt of L-glutamic acid. L-glutamic acid is one of the most numerous non-essential amino acids in nature. Kikunae Ikeda (1864-193) isolated this compound from a seaweed in 1908 as a new taste substance. He named this taste umami (sometimes known as the fifth taste). It is mainly used as a food additive to enhance flavors, as it highlights, combines and balances the character of other flavors.
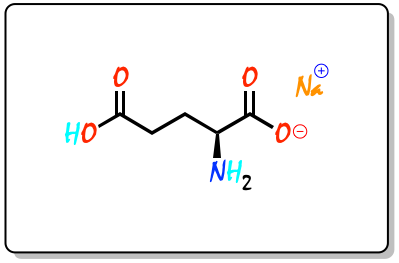
The systematic name of the IUPAC for E621 is sodium (2S)-2-amino-5-hydroxy-5-oxo-pentanoate, or also: sodium salt of L-glutamic acid.
Chemical structure
E621 has the molecular formula C5H8NNaO4. Since it is an amino acid, it has the corresponding functional groups –NH2 and -COOH. In addition, at the other end of the molecule (carbon chain) it has another acid group -COOH. Moreover, as it is a sodium salt, one of the acid groups is ionized.
 |
| 3D Structure |
Physicochemical properties
It is a white or dirty gray crystalline solid, odorless (odorless). It is very soluble in water (solubility greater than 100 g/L). It has a molecular mass of 169.111 g/mol. Its melting point could not be determined because it decomposes at 225 ºC and has a density of 2.1 g/cm3. E621 generally crystallizes with one water molecule (monohydrate). In solution it dissociates into glutamate ions and sodium ions, is not hygroscopic and is insoluble in organic solvents. It is generally stable under food processing conditions. E621 does not decompose during cooking and, like other amino acids. In the presence of sugars and at very high temperatures, it produces the Maillard reaction (browning reaction).
Industrial production
Kikunae Ikeda isolated glutamic acid in 1908 from kombu seaweed (Laminaria japonica) by extraction and crystallization in water.
E621 has been produced by three methods:
- Hydrolysis of plant proteins with hydrochloric acid to break peptide bonds (1909-1962).
- Direct chemical synthesis with acrylonitrile (1962-1973)
- Bacterial fermentation (present).
Bacterial fermentation is the current method employed to produce glutamic acid.
Wheat gluten was originally used for hydrolysis because it contains a very high percentage. As the demand for E621 increased, other methods such as chemical synthesis and fermentation were evaluated. Because the polyacrylic fiber industry was developed in Japan in the mid-1950s, acrylonitrile was used as a starting material to synthesize E621.
Currently, most of the global production is carried out by bacterial fermentation (a process similar to the production of vinegar or yogurt). Sodium is added later, for neutralization. During fermentation, Corynebacterium species, cultured with ammonia and sugars from sugar beet, sugar cane, tapioca or molasses, excrete amino acids into a culture broth from which L-glutamate is isolated.
References
, “On the taste of the salt of glutamic acid” Proceedings of the 9th International Congress in Applied Chemistry, Section VIII c: Bromatology, 1912, Vol. 38