What is elimination (E1 and E2)?
As in substitution reactions, elimination reactions follow two distinct mechanisms called E1 and E2.
Elimination E1
In reaction E1, the reaction rate depends only on the substrate concentration. The mechanism of the reaction is similar to that of SN1, with the difference that the intermediate carbocation evolves towards the formation of an alkene by loss of a hydrogen in the position next to where the positive charge is located.
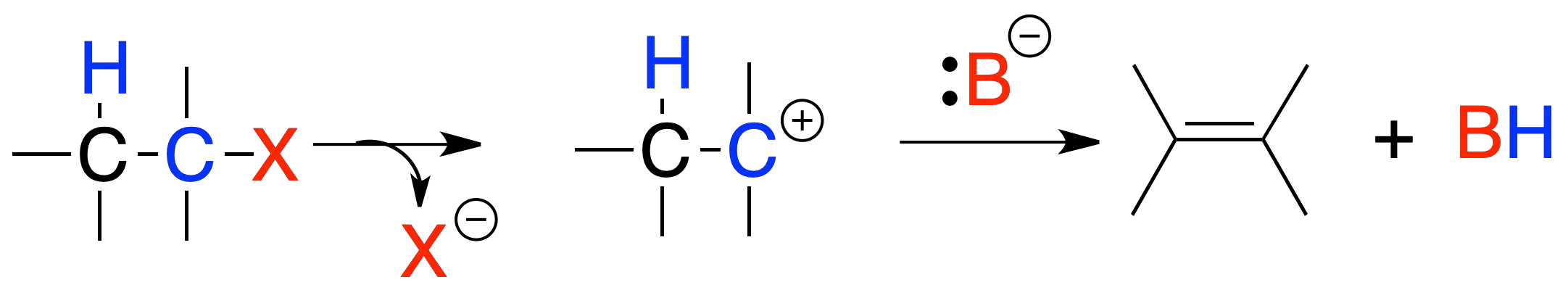
The reaction is favored by the formation of stable carbocations, good leaving groups and weak bases. It follows that the order of reactivity is tertiary > secondary >>> primary. In addition, the most stable alkene (the most substituted) is usually formed and if possible the trans isomer predominates over the cis isomer.
Elimination E2
In reaction E2, treatment of a primary or secondary alkyl halide with a strong base leads to the formation of an alkene. The reaction rate depends on the concentration of the substrate and the base.
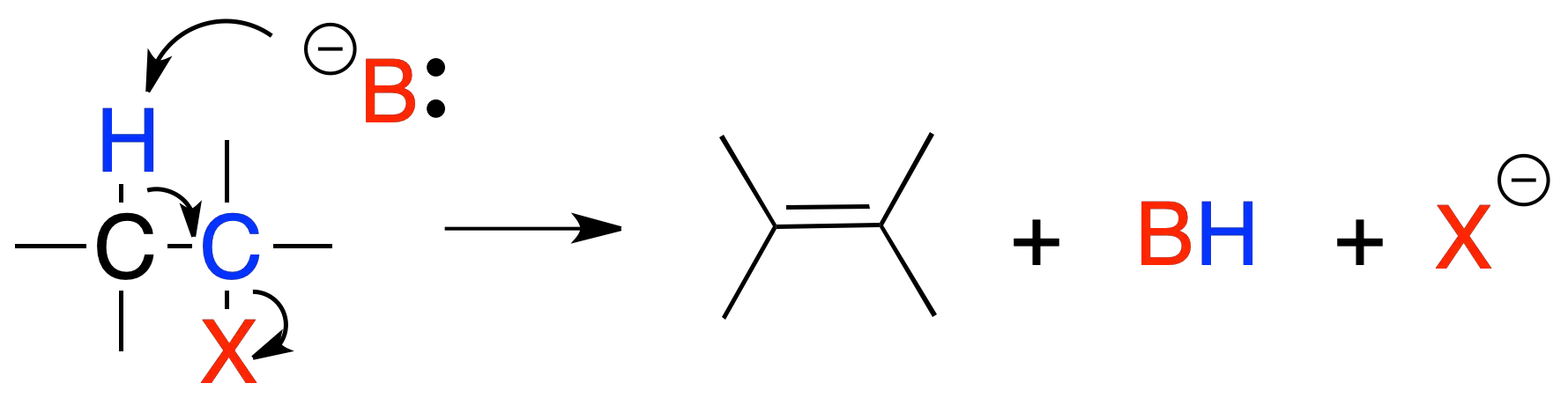
The proposed mechanism is a concerted process and the groups to be eliminated, X and H, are arranged in an antiperiplanar manner.
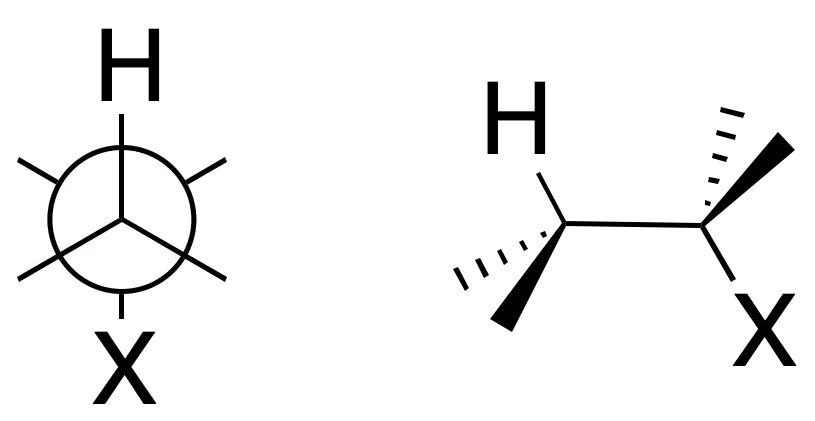
The process is regioselective, since in the elimination the most substituted alkene is predominantly produced (Zaitsev’s rule). If there is the possibility of formation of cis and trans isomers, the formation of the trans alkene predominates over the cis alkene.
If a bulky base is used (the typical case is potassium tert-butoxide), the least substituted alkene is mostly formed (Hofmann’s rule), since steric factors prevail.
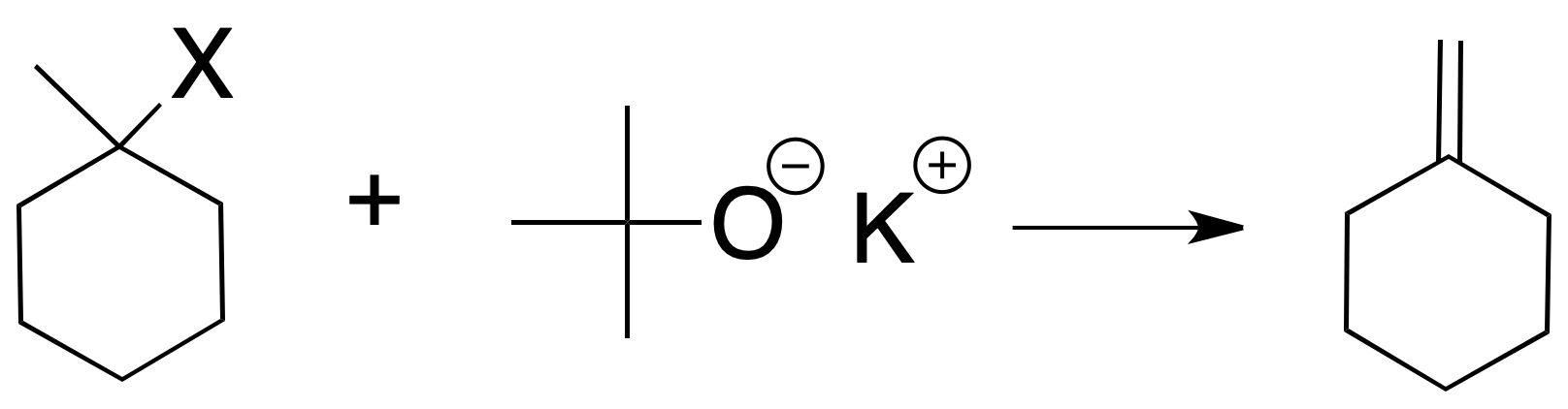
Other things being equal, the rate of the reaction depends on the nature of the halogen, according to this sequence I > Br > Cl >> F. The reaction is incompatible with base-sensitive groups.
The substitution and elimination reactions are competitive, and an important factor is temperature. At high temperatures, elimination is favored.