What is an enantiomer?
An enantiomer refers to two structures that are mirror images but not superimposable. The term comes from the Greek enántios, “opposite”, and méros, “part” or “portion”. They are also called optical isomers.
Characteristics
The main characteristic of two enantiomers is that all their physicochemical properties are the same except that they bend the polarized light in a plane of different shape. One of the isomers bends the plane of polarization to the right (R) and the other in the opposite direction (S). For all these reasons, these enantiomers are also called optical isomers.
Therefore, molecules with one stereocenter (e.g. an asymmetric carbon) are always optically active (chiral, see chirality). But when, such molecules present more than one stereocenter this is not always true. For example, in the case of meso forms. On the other hand, achiral molecules, without stereocenters, are optically inactive.
Also, a solution with stoichiometric mixtures of each enantiomer is optically inactive and is called a racemic mixture.
To measure the specific rotation of polarized light, a polarimeter is used. This physical property is characteristic of the structure of each enantiomer, its concentration and the solvent used in the measurement.
[α]D25°C
The value of [α]D, measures the specific rotation, and indicates the enantiomeric composition of the sample.
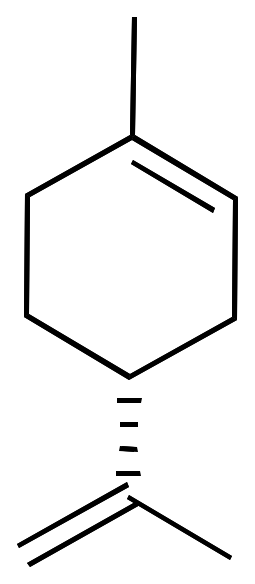
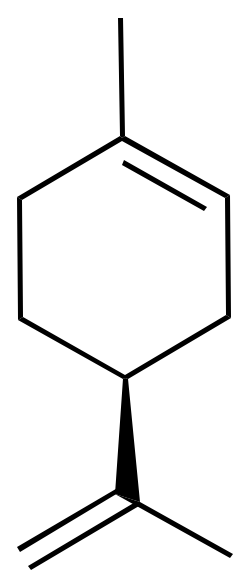
For example, limonene has an asymmetric carbon as a stereocenter. Consequently, there are two optical isomers: the enantiomer R-limonene and S-limonene. They are also called D-limonene and D-limonene (formerly called dextro and levo, respectively).
 |
 |
| 3D Structure | 3D Structure |
R-limonene is obtained commercially from lemon and exhibits a chiral rotation, [α]D, of 87–102°. R-limonene is obtained commercially from lemon and exhibits a chiral rotation, [α]D, of 87–102°.
With a chiral center
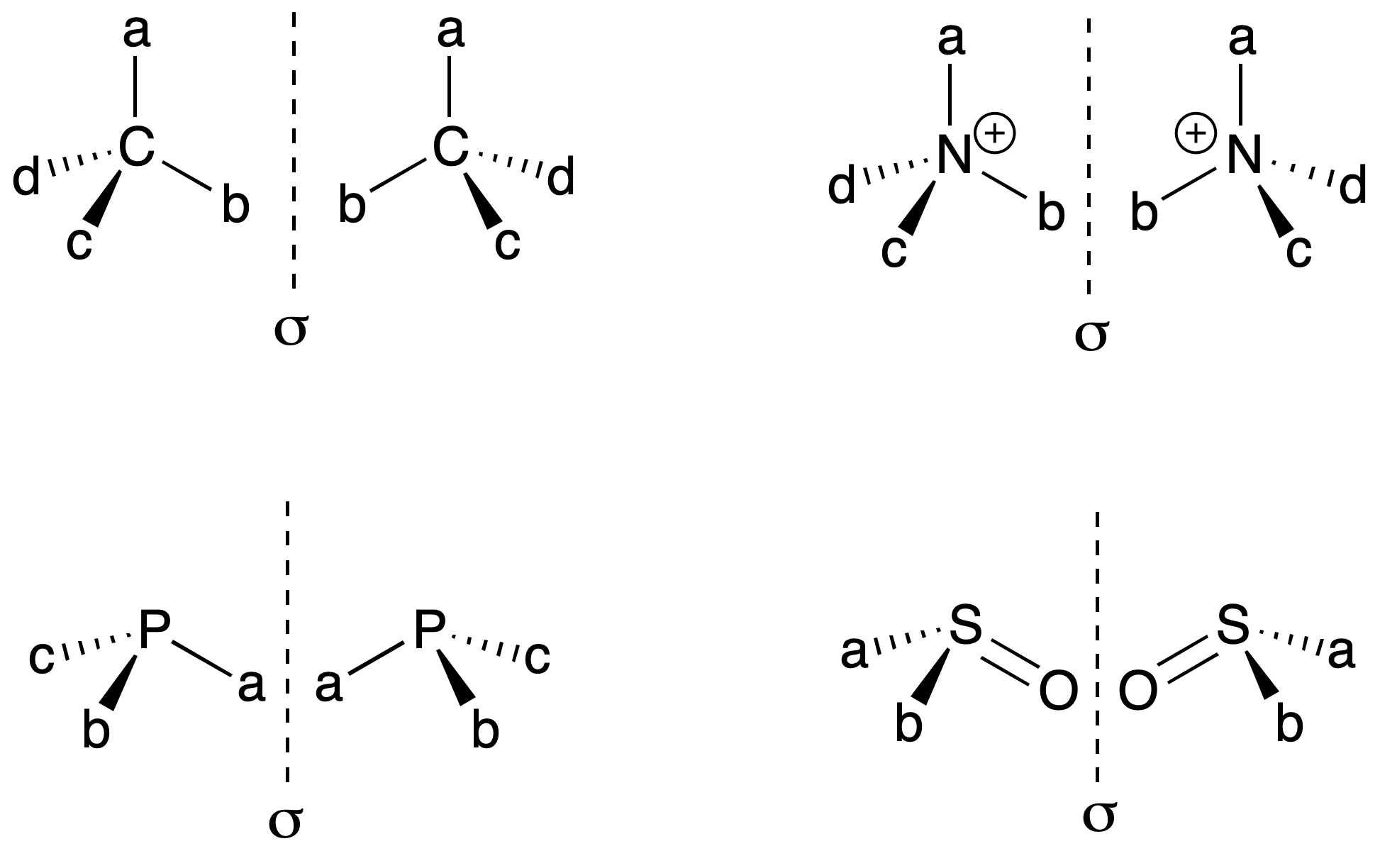
It is identified with an atom that has a set of substituents in a spatial arrangement that is not superimposable on its mirror image. Carbon bonded to four different substituents (Cabcd), is called asymmetric carbon (see chirality). However, there is also the possibility with a nitrogen atom attached to four different substituents (N+abcd), phosphorus with three different substituents (Pabc), sulfur (SOab), etc.

In the case of nitrogen with 3 substituents (Nabc), there is rapid interconversion between the two possible enantiomers. Consequently, the possibility of stereoisomers is eliminated.
Thus, each of the enantiomers is assigned a corresponding letter, R or S, which comes from the Latin rectus (right) or sinister (left), respectively. Samples with only one of the two enantiomers are referred to as enantiopure compounds.
Without a chiral center
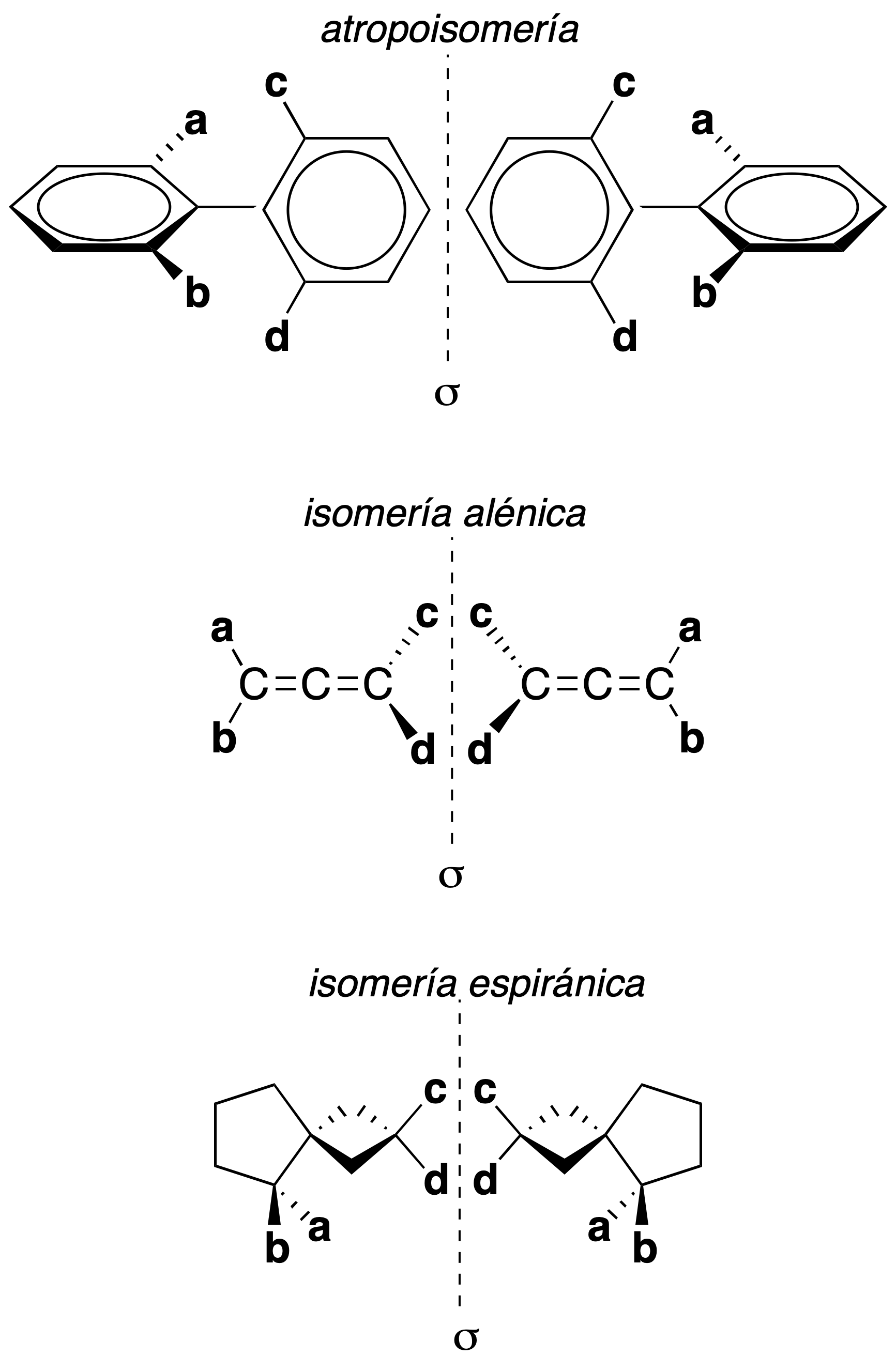
A chain of four non-coplanar carbon atoms, or rigid groups in a stable conformation which, by imaginary or restricted rotation about the central atom, lead to stereoisomers, give rise to three types of stereoisomerism without a chiral center as shown in the figure.