Objective
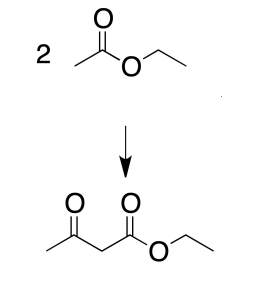
The objective consists of performing a Claisen condensation between two molecules of an ester (ethyl acetate) to form a β-ketoester (ethyl acetoacetate).
Background
Ester enolates undergo addition-elimination reactions with other ester functions giving rise to β-ketoesters. These transformations, known as Claisen condensations, can occur between the same or different ester molecules. The formation of enolates involves the use of strong bases compatible with the esters used.
Experimental procedure
Reaction of ethyl acetate
In a 250 ml round bottom flask 50 g (55.5 ml) of pure ethyl acetate are placed and 5 g of sodium wire, previously debarked, are added. A reflux condenser is adapted to the flask and a drying tube is placed at the upper end of the flask. The reaction mixture is heated to reflux, so that the ethyl acetate is kept in gentle boiling.
If the ethyl acetate contains only a small amount of alcohol or water, a violent reaction will not occur immediately and a slight release of hydrogen will be observed.
Heating is continued until the sodium is completely dissolved (approx. 3 h). Prolonging the reflux too long will result in a decrease in performance.
After this time the heating plate is switched off and while the reaction crude is still hot, a mixture consisting of 14 ml of acetic acid in 16 ml of water is added little by little with occasional stirring until acid pH is reached.
Isolation
Subsequently, an equal volume of cold saturated brine is added to the mixture and extraction is carried out with the separating funnel. The organic layer (upper) consists of a mixture of acetic acid (minor), ethyl acetate and ethyl acetoacetate (major).
To separate the ethyl acetoacetate, it is washed in the separating funnel with a saturated solution of sodium bicarbonate NaHCO3 and the organic layer.
Purification
The separated ethyl acetoacetate is purified by simple distillation. The distillation is stopped when the temperature reaches 95 ºC. The residue is distilled under vacuum. The first (relatively small) fraction consists of ethyl acetate and water. In the second fraction, which distills in a few degree range, ethyl acetoacetate is obtained.
Lower distillation temperatures than indicated can be observed depending on the pressure reached by the vacuum system used, for example, at 14 mmHg 74 ºC, at 29 mmHg 88 ºC, at 45 mmHg 94 ºC and at 80 mmHg 100 ºC.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
| Ethyl acetoacetate | 130.14 | -43 | 181 | 1.029 |
| Na | 22.99 | 97.8 | 883 | 0.968 |
| NaHCO3 | 84.01 | 300 | - | 2.160 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic acid |   |
| Ethyl acetate |   |
| Ethyl acetoacetate |  |
| Na |   |
| NaHCO3 | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
| Ethyl acetoacetate | XYIBRDXRRQCHLP-UHFFFAOYSA-N |
| Na | KEAYESYHFKHZAL-UHFFFAOYSA-N |
| NaHCO3 | UIIMBOGNXHQVGW-UHFFFAOYSA-M |
Video on ethyl acetoacetate synthesis
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145