What is fractional distillation?
Distillation is one of the most widely used techniques to purify liquids in the laboratory and nearly the entirety of all the processes of industrial separation.
La fractional distillation uses multiple cycles of vaporization-condensation and is used to separate components of liquids in your boiling point differ by less than 25 ºC. Each one of the components to be separated are referred to as fraction. The assembly is analogous to the simple distillation with the difference between the round-bottomed flask and the head of distillation inserts a rectification column (you can submit deferent types of design: column vigreux column padding, etc.). This rectification column is usually filled with glass beads or steel wool, which provides a large surface area for the fluid to condense and re-evaporated several times depending on the design.
When the mixture is heated, the steam rises and surrounds the hollow of the rectification column, and enriched it with the component is more volatile, while the liquid falls down, passing through the spine into the round-bottomed flask, to break in the component is less volatile and causing thereby a separation more efficient. The overall process is equivalent to performing multiple distillations simple mixture.
Mounting of fractional distillation
- The clamp and the nut is fixed to the grid of the laboratory, on the table or range hood.
- The round-bottomed flask is placed on a heater plate, adjusting a clip to the neck and to the grid.
- Mounts to the column vigreux in the neck of the flask round bottom and is secured with a clamp and nut to the grid.
- Place the head of the distillation column vigreux, with a thermometer with its adapter by regulating the height of the bulb.
- With another clamp and nut to the lattice adjusts the coolant straight to the head of distillation, and the ends of the capacitor connecting the head and tail of distillation are secured with two clips (one at each end).
- Connect the jacks of water from the refrigerant so that the water flow from the lower end toward the head of distillation (top end).
- Place a Beaker as a collector under the adapter distillation, the height can be adjusted with the use of an elevator.
Mounting schematic diagram
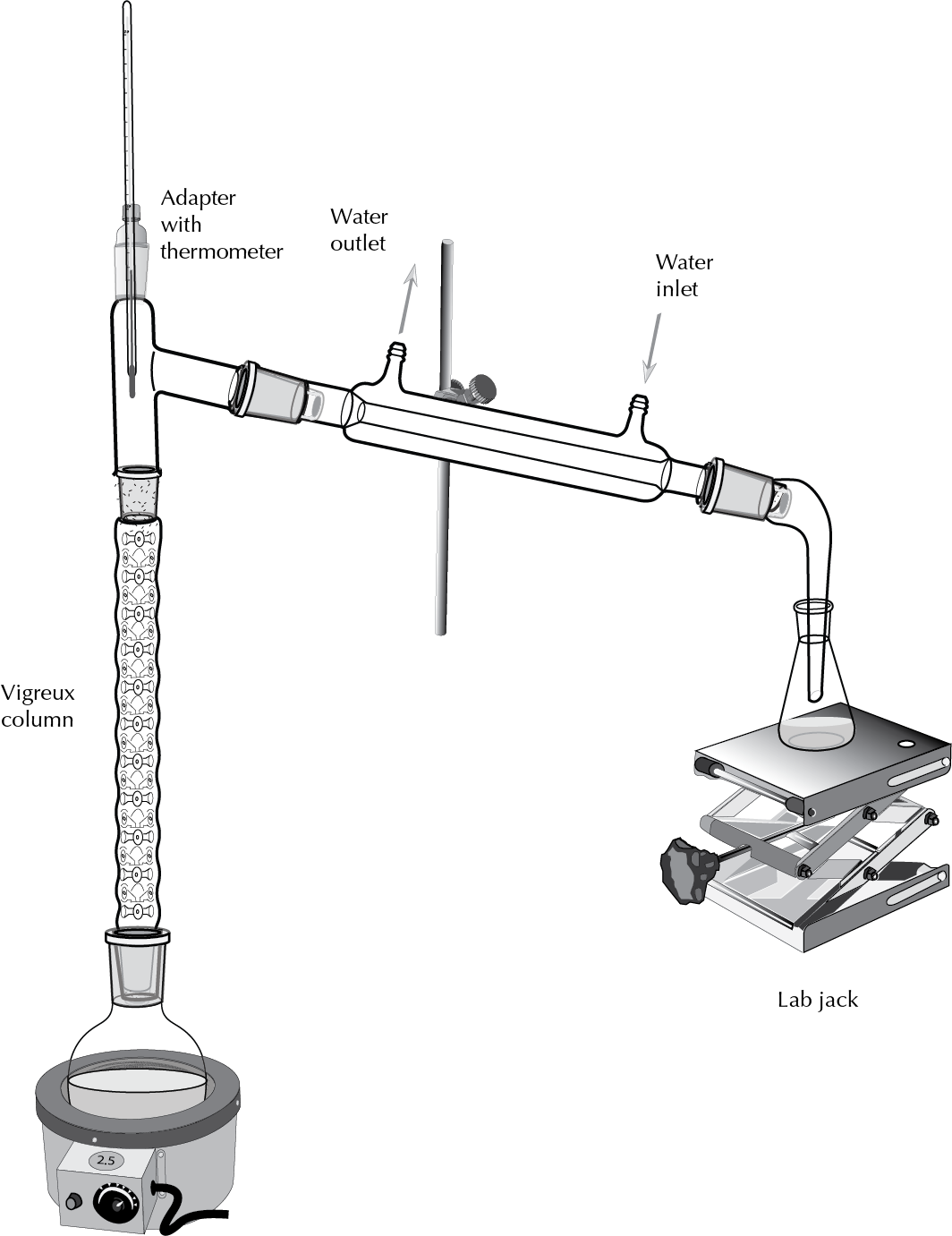
As the vapors to ascend the rectification column (column vigreux), forms a ring of condensation rises slowly as the vapors are separated. When the vapor reaches the top of the column, the molecules are more volatile passed to the coolant rectum where it is collected as a distillate of very pure.
The basis of the fractional distillation
The fundamentals of fractional distillation can be displayed through a curve distillation as shown in the figure. Thus, the distillation of an equimolar mixture of compounds To + B is described by the curve of distillation.
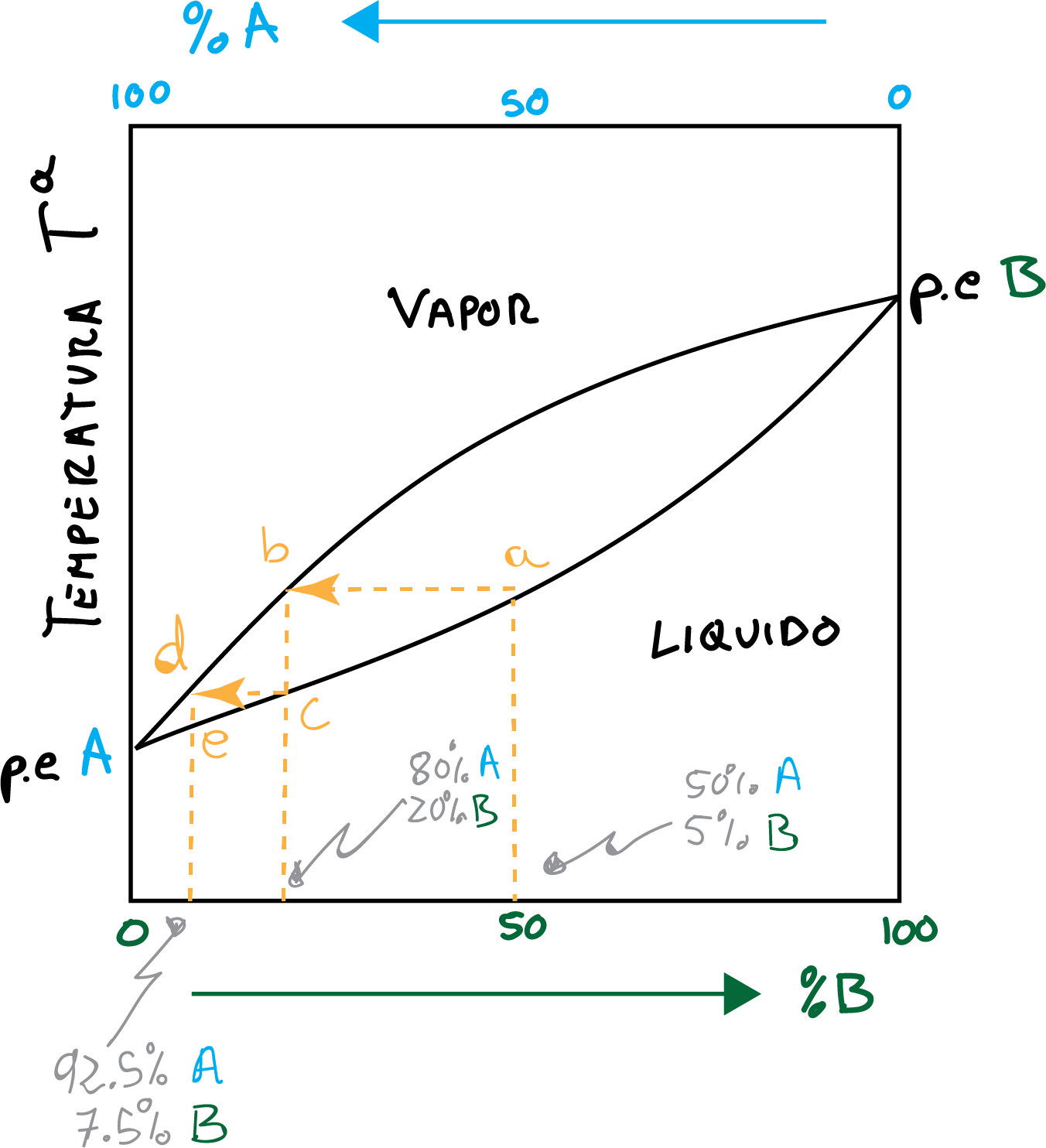
An event of vaporization-condensation is represented in the Figure according to the points a → b → c. This process represents a dish theoretical and produces a distillate that is 80% To 20 % B.
A second event of vaporization-condensation is represented following the points c → d → e, and produces a distillate that is 92.5 % To 7.5 % B.
In conclusion, a 50 % / 50 % mixture of two components whose boiling points differ only in 20-30 ºC
it would require at least three theoretical plates to obtain a distillate with more than 92.5 % purity.
In practice, the distillations fractionated often produce something of mixtures. The best way to get purity through fractional distillation is when initially the samples show very little impurity.
Types of rectification column (or fractionation)
The choice of what to rectification column to use will depend on the application and in part on the availability and the task in question. On the market there are different models and the most popular are summarized in the figure:
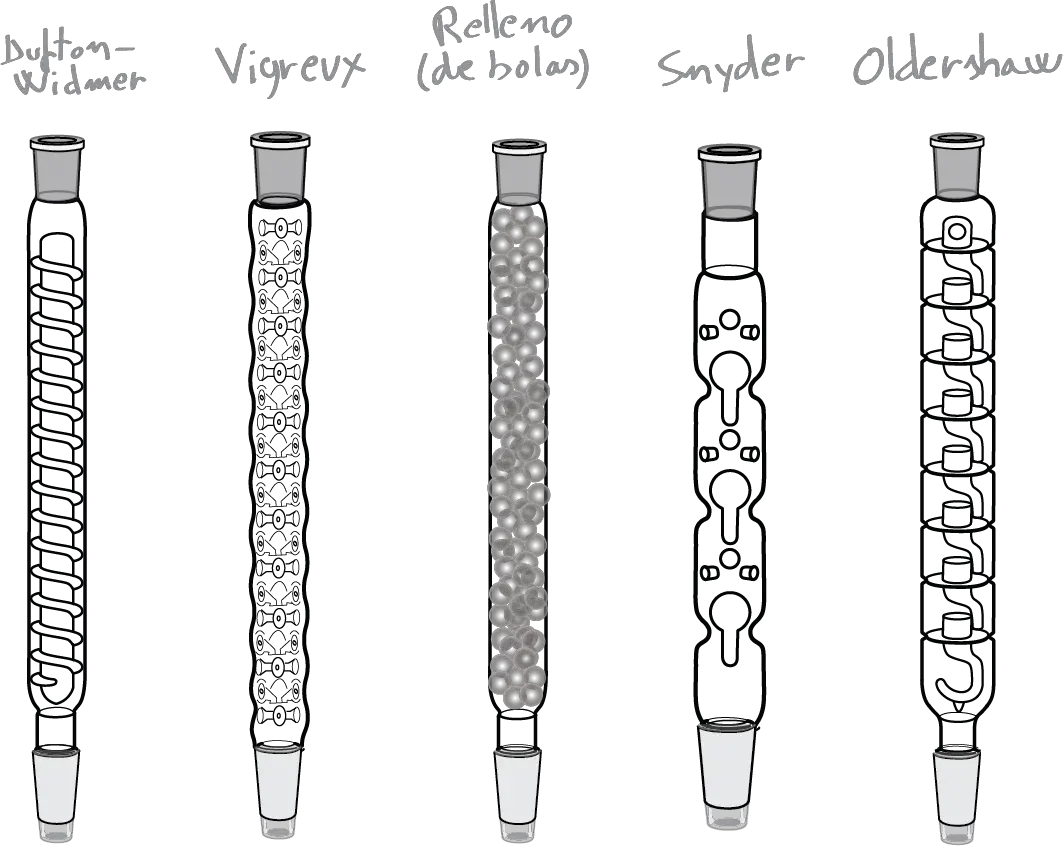
- Column Vigreux: This is a modification of the condenser air. It has a series of deep indentations and pointed in its side wall where the vapors are condensed while passing through the column, and that serve to increase the surface of contact between the steam and the condensate.
- Column Snyder: Is a type of column is very effective which contains a series of segments which, in turn, have a glass sphere floating in order to increase the mixture of steam and condensate.
- Packed columns: These are air condensers that are stuffed with small pieces of glass or glass Raschig rings ceramic (Parts tube length and diameter are similar; its name comes from the inventor, the German chemist Friedrich Raschig).
To keep the filling in these columns, there is usually some type of constriction that allows the passage of vapor but prevents you from falling into the interior of the flask. - Other columns grinding machines, such as the Overshaw as indicated in the figure.
The most used is the column Vigreux with incisions of glass, the column of Dufton-Widmer, the Oldershaw, of Snyder, the fill of balls, etc
The designs of these columns have different surface areas and numbers of theoretical plates and, therefore, differ in their ability to separate components of boiling point nearby.
Also, they differ slightly in the amount of compounds less volatile that is retrieved in the liquid that condenses. The column Vigreux has the smaller surface area, so that it is less effective at separating components. However, even with a low surface area, has a higher recovery, so that is the optimal choice if a separation is not particularly difficult.
The packed column of balls exhibit a high surface area, being a good option for the separation of components of boiling point. However, these columns present the greatest loss of material.
Examples
The fractional distillation can be used to purify reactants and products.
The cyclopentadiene (boiling point 39-43 ° C) is used in many reactions, such as polymerization and in the Diels-Alder reaction.
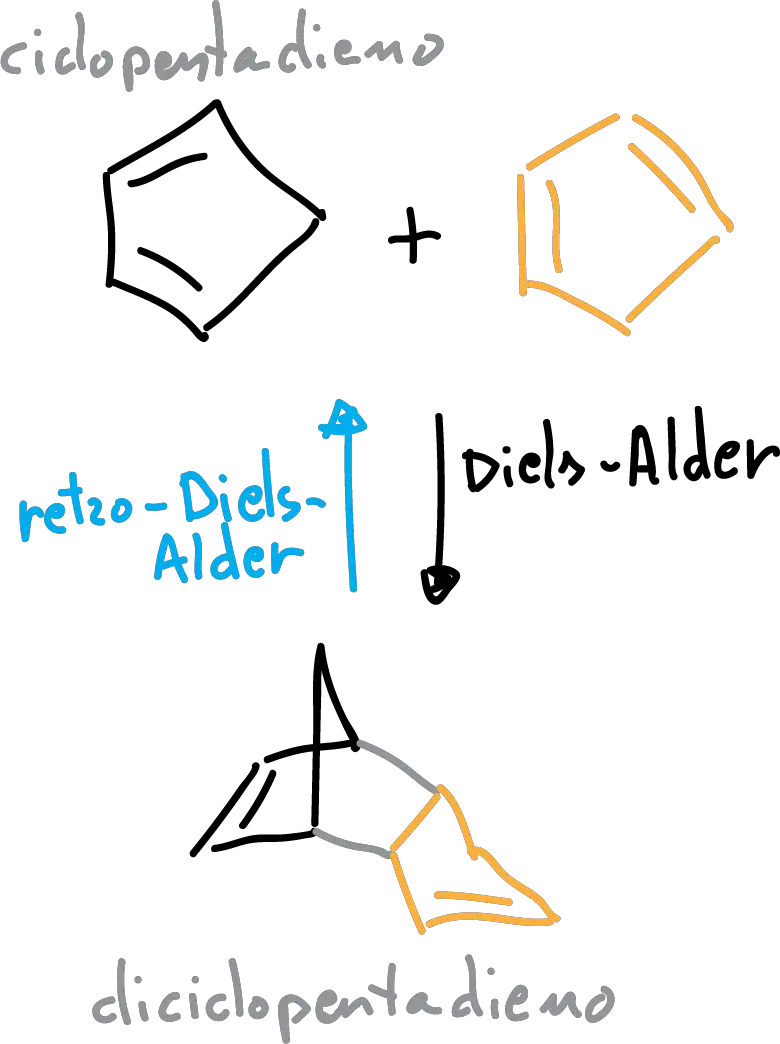
However, it is so reactive that it cannot be stored because it undergoes a reaction Diels-Alder himself, in order to form dicyclopentadiene . Therefore, the cyclopentane is generated in situ when you need to, from the diciclopentadieno commercial. Using distillation with rectification the diciclopentadieno when heated gives a reaction of retro-Diels-Alder(reverses the reaction of dimerization) and is fragmented in cyclopentadiene.
The distillation can be used to remove the monomer (cyclopentadiene), as they form. The two components (monomer and dimer) of the crude reaction have boiling points are very different, and, in principle, we could think that it would be a simple distillation. However, the temperature required for the reaction retro-Diels-Alder reaction is close to the boiling point of the diciclopentadieno (170 ° C). In consequence its vapor pressure cannot be ignored. So it is necessary to a fractional distillation in place of distillation simple for this process and to completely separate the cyclopentadiene the dicyclopentadiene.
Video about Fractional distillation
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2