What are imidazoles?
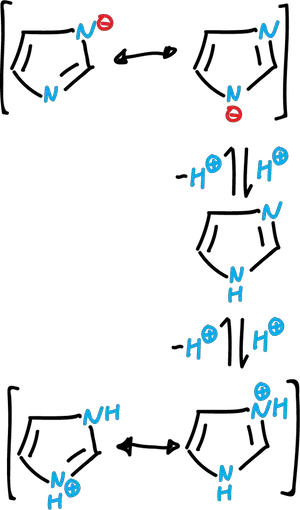
The imidazoles are nitrogenated heterocycles, with two nitrogens in positions 1 and 3. They are planar and have a significant resonance energy, somewhat higher than that of the pyrrole.
The imidazole system can act both as a base and as an acid. Free imidazole is a moderately strong organic base (pKa7.0). It can also act as a weak acid (pKa 14.5). Both the cation and the anion have symmetrical delocalized structures.
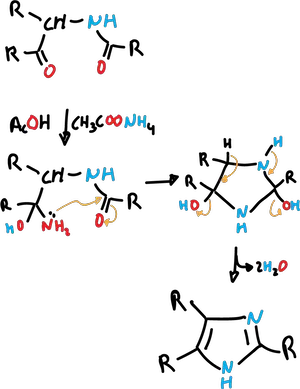
Synthesis of imidazoles by cyclization of 1,4-dicarbonyl compounds
The imidazole synthesis is carried out between 1,4-dicarbonyl compounds and appropriate functions. This process is analogous to the Paal-Knorr synthesis of pyrroles.
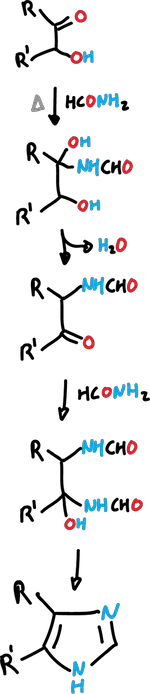
Bredereck imidazole synthesis
Bredereck imidazole synthesis is performed when an α-hydroxyketone (or α-haloketone) is heated with formamide (HCONH2).
Mackwald synthesis
It involves the reaction of α-aminoketones with thiocyanates (or isothiocyanates) to yield imidazole-2-thiols,
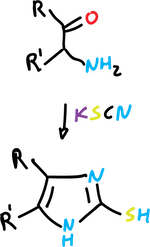
and with cyanamide to give 2-aminoimidazoles.
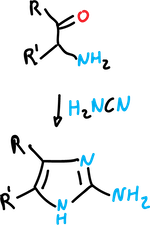
Imidazole reactions
Imidazole is an excellent nucleophile and can easily racate in nitrogen with alkylating and acylating agents.
N-acylation
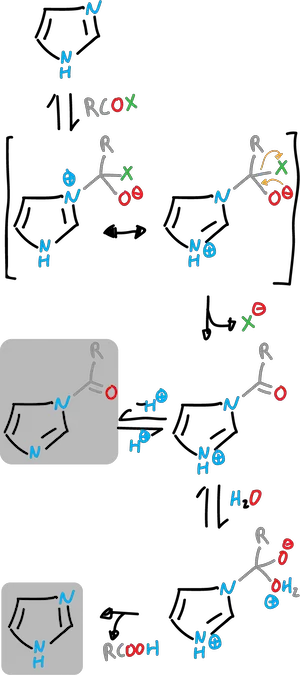
Imidazoles can be N-acylated by treatment with acyl halide in an aprotic solvent.
The N-acylimidazoles are very useful “acyl transfer” reagents and their reactivity is comparable to that of acid halides or anhydrides.
Acylimidazoles undergo typical addition-elimination reactions, for example, with alcohols to give esters, amines to give amides and Gridnard reagents to give ketones.
Also, imidazole can catalyze the hydrolysis of esters and other acyl derivatives.
With the acylderivatives (R—COX) in which X is a good leaving group, the imidazole acts as a nucleophile attacking the carbonyl group.
N-alkylation
N-alkylation is carried out in the presence of base. This is done in such a way that the species being rented is the anion.
Alkyl halides and similar compounds can be used.
Under neutral conditions additional alkylation and formation of quaternary salts may occur.
The C4-substituted imidazoles give two possible N-alkylation products. This will depend on the alkylation conditions and the steric and electronic effects of the substituent.
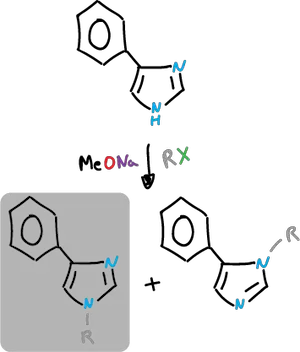
The secondary product can be obtained by acylating (quaternizing) it and then removing the acyl group.
Reactions at the carbons of the imidazole ring
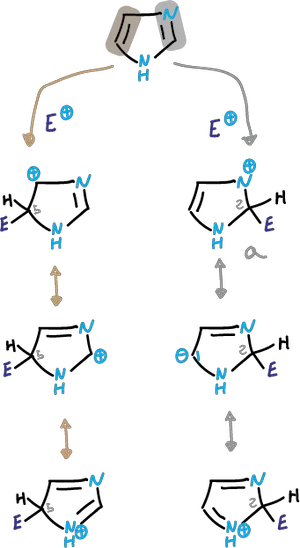
These reactions are those one would expect from a stable aromatic heterocycle. It is less activated for electrophilic attack compared to pyrrole or thiophene.
The electrophilic substitution is complicated by the fact that N3 is protonated in the acidic medium.
Depending on the conditions, the reaction can take place in the neutral imidazole or in an imidazole cation.
In reactions that take place in strong acid, the cation that is deactivated against electrophiles participates.
Friedel-Crafts Alkylation and Acylation
Thus, the alkylation or acylation of Friedel-Crafts almost always fails.
In cases where substitution occurs (difficult nitration and sulfonation), it is carried out on the conjugated acid obtained in the first protonation step, and is carried out on the C4 carbon.
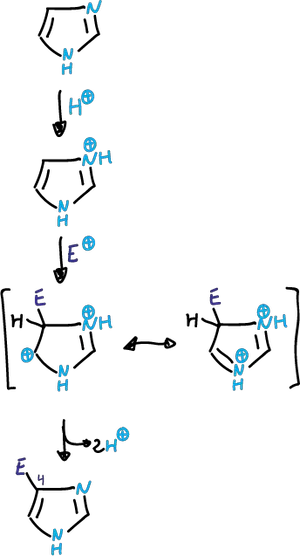
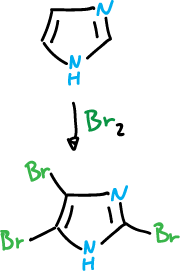
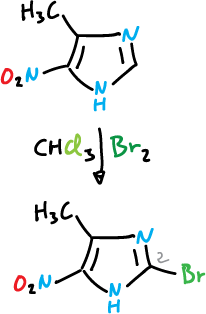
Bromination
Bromination takes place at the C4 position and is carried out with organic solvents and in the absence of catalyst.
One would think that the substitution at position C2 would be favorable relative to C5.
However, structure (a) has charge that has to delocalize through the imine nitrogen and is therefore very unstable. However, with bromine the imidazole reaches 2,4,5-tribromo-imidazole, in the absence of catalyst.
It also undergoes bromination at the C5 position when the C4 position is occupied by an electron-donating substituent.
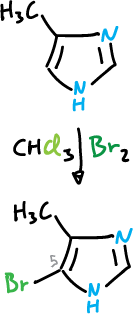
When both the C4 and C5 positions are occupied, substitution at C2 often occurs.
Hydrogen elimination at C2
An exceptional aspect in the chemistry of imidazoles is the ease with which they can lose the proton at C2 under neutral or basic conditions.
This process appears to involve the formation of an (a) imidazolium ylide.
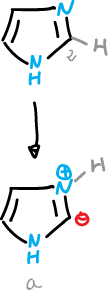
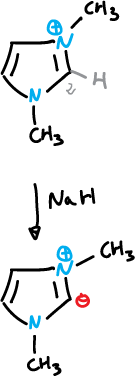
Imidazolium ylide can be generated from the 1,3-dimethyl imidazolium cation, and then substituted at C2 with alkyl halides and other electrophiles.
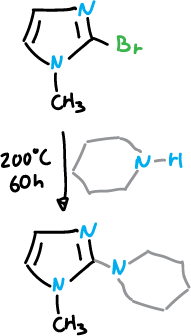
Nucleophilic substitution reactions
Nucleophilic substitution reactions in imidazoles are most easily ligated at C2, but are generally difficult at any position unless additional activating groups are present.
The imidazole ring is cleaved under strongly basic conditions making certain displacement reactions with basic nucleophiles impossible.
For example with piperidine.
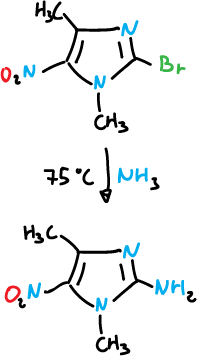
Or also with ammonia.