What is isopropanol?
Isopropanol (IUPAC name propan-2-ol), also called isopropyl alcohol or 2-propanol. It is a secondary alcohol with the following physicochemical properties: flammable, transparent and with a strong odor.
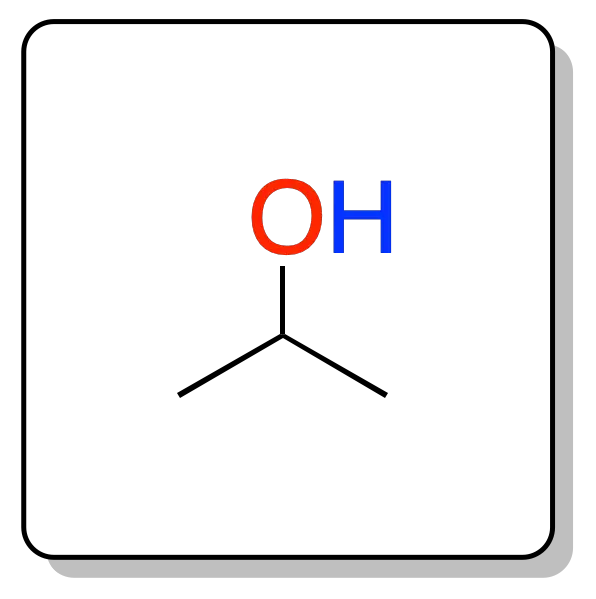
Its chemical formula is CH3-CH(OH)-CH3, formed by the union of a hydroxyl group -OH with an isopropyl group.
 |
| 3D Structure |
Isopropanol applications
Isopropanol is used in the manufacture of a wide variety of industrial and household products and is used as an ingredient in detergents, disinfectants, etc.
Physico-chemical properties
It is miscible in water, ethanol (EtOH), chloroform and ether. It dissolves cellulose, various oils, polyvinyl bromide (PVB), natural resins, etc. Unlike EtOH and methanol (MeOH), it is not miscible in salt solutions and can be separated from aqueous solutions by adding salt (e.g. sodium chloride, NaCl).
It forms an azeotrope with water, which is characterized by a boiling point of 80.37 ºC, and has a composition of (91 % v/v) in isopropanol. It is not suitable for human consumption. Its melting point is -89 ºC. It absorbs in the UV/vis at a value of λmax = 205 nm.
Reactivity
It exhibits the alcohol reactions characteristic of this -OH functional group. Isopropanol is oxidized to give acetone. The reaction is carried out with oxidizing agents such as chromic acid. It can also be carried out by heating with copper catalysts in a dehydrogenation reaction.
CH3-CH(OH)-CH3 → CH3-C(O)-CH3 + H2
It is frequently used as both solvent and hydrogen source in the Meerwein-Ponndorf-Verley reduction reaction, and other hydrogen transfer reactions.
It reacts with active metals such as potassium to give alkoxides (isopropoxides). For example, the reaction with aluminum, initiated with traces of mercury, is used to prepare aluminum isopropoxide, used as a catalyst.
It can be brominated with phosphorus tribromide (PBr3) to give 2-bromopropane. También, se pueden llevar a cabo otras reacciones de hidrogenación. It undergoes dehydration reactions to give the corresponding alkene (propene) if heated with sulfuric acid (H2SO4).