What is Lassaigne’s test?
Lassaigne’s test (sodium fusion test) is one of the most widely used methods (in qualitative organic qualitative analysis) for the determination of nitrogen in organic compounds. In addition, it can be used for the determination of sulfur, halogens and phosphorus.
Briefly, in the Lassaigne’s, the substance to be examined is melted with sodium, whereupon the elements, previously mentioned, to be identified will form the corresponding derivatives. These are: sodium cyanide NaCN, sodium sulfide Na2S, sodium halide NaX, and sodium phosphate Na3PO4 (after treatment with nitric acid HNO3), respectively. These compounds are then extracted with boiling water and identified.
Procedure
A lentil-sized piece of sodium is placed in a Pyrex® test tube and heated over a flame until the sodium melts. About 200 mg of the test substance is then carefully added several times and the tube is heated to cherry red. The tube is allowed to cool and 2 ml of EtOH is added, when the bubbling stops about 10 ml of deionized water is added, it is heated to boiling and filtered. With the obtained solution several fractions are made in which we are going to identify the present ions.
Nitrogen and sulfur recognition
Procedure:
- To about 3 ml of the alkaline filtrate, obtained in the previous section some crystals of ferrous sulfate are added, it is boiled smoothly during some seconds and the solution is allowed to cool. It is then acidified with dilute HCl (or dilute H2SO4). The appearance of an intense blue coloration (or suspension) (due to Prussian blue) indicates the presence of nitrogen.
- To 1 ml of the filtrate solid sodium bicarbonate is added until saturation. One or two drops of this solution are added to 1 ml of a 1 % solution of p-nitrobenzaldehyde in DMSO (see list of acronyms). The appearance of a purple color indicates the presence of nitrogen. A green color indicates the presence of sulfur. If both are present only the purple color appears, so the presence of sulfur should be confirmed by the lead acetate assay.
Sulfur recognition
- Procedure A) lead acetate assay. About 2 ml of alkaline solution is acidified with acetic acid and a few drops of lead acetate solution are added, the appearance of a black precipitate of lead sulfide will indicate the presence of sulfur in the substance.
Pb(CH3COO)2 + Na2S → PbS↓ + PbCH3COONa
- Procedure B) About 2 ml of alkaline solution is acidified with HCl and heated gently. Over the mouth of the test tube a paper impregnated with lead acetate is placed. The appearance of a blackish-brown stain of lead sulfide will indicate the presence of sulfur.
Na2S + 2H⊕ → H2S ↑ + 2Na⊕
Pb(CH3COO)2 + H2S → PbS↓ + PbCH3COOH
Nitrogen recognition
This procedure requires the prior elimination of sulfur.
- Procedure: To about 3 ml of the alkaline filtrate add a few crystals of ferrous sulphate, boil gently for a few seconds and allow the solution to cool. It is then acidified with dilute HCl (or dilute H2SO4). The appearance of an intense blue colouring (or suspension) (due to Prussian blue) indicates the presence of nitrogen.

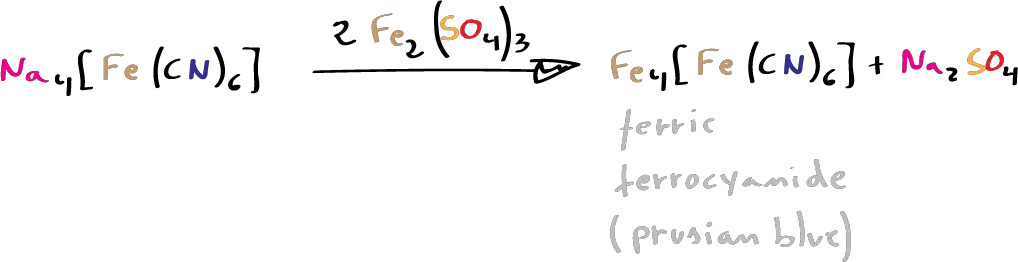
Recognition of halogens (Beilstein test)
Procedure: A copper wire is heated to red heat until it does not give color to the flame. The wire is allowed to cool and a small amount of test sample (or alkaline solution) is taken and the wire is heated to the flame again. The appearance of a green flame indicates the presence of halogens.
Recognition of halogens (silver halide assay)
Procedure: About 3 ml of alkaline solution is acidified with concentrated HNO3. If the sample contained sulfur and/or nitrogen the solution is boiled for a few minutes to remove the hydrogen sulfide and hydrocyanic acid formed. Then 4-5 drops of silver nitrate solution are added. The formation of a white or yellow precipitate that darkens rapidly in the light indicates the presence of halogens. If the precipitate is white and once decanted it dissolves in ammonia it is chloride, if it is pale yellow and hardly soluble it is bromide and if it is strong yellow and completely insoluble in ammonium hydroxide it is iodide.
Fluorine recognition
A portion of the original sodium fusion filtrate is acidified with dilute hydrochloric acid HCl, and treated with drops of zirconium-alizarin reagent. The presence of fluoride ions is indicated by the disappearance of the violet color of the reagent and appearance of a yellow color of the alizarin. Care should be taken not to add too much reagent.
Preparation of zirconium-alizarin reagent
Dissolve 0.5 g of alizarin in 50 ml of water, then add 1 g of zirconium nitrate, Zr(NO3)4, dissolved in 50 ml of hydrochloric acid HCl (5 %). The mixture is dissolved with water, to a final volume of 150 ml.
Phosphorus recognition
A portion of the sodium fusion filtrate obtained from the original sample is boiled with an excess of concentrated nitric acid. Subsequently, it is allowed to cool and an equal volume of ammonium molybdate solution (NH4)2MoO4 is added. Finally, it is heated to 50 °C and allowed to stand. The presence of phosphorus is confirmed by the formation of a yellow precipitate.
References
- Lassaigne (1843) “Mémoire sur un procédé simple pour constater la présence de l’azote dans des quantités minimes de matière organique” [Memorandum on a simple method for determining the presence of nitrogen in small amounts of organic matter] Comptes rendus, 16, 387-391.
- Jean Louis Lassaigne Abrégé élémentaire de chimie inorganique et organique [A Basic Compendium of Inorganic and Organic Chemistry] Labé (Paris) 1846
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145