The generic name of the aromatic hydrocarbons mono-and polycyclic is arenes and the radicals derived from these are called radical aryl. The first term of the series is the benzene (C6H6) (recommendation A-11.3). When on the benzene ring there is a substituent, the hydrocarbon is named by prepending the name of the radical, followed by the word benzene (recommendation A-12-2).
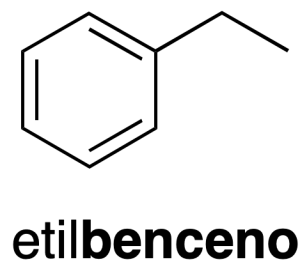
The radical monovalent benzene C6H5– it is called phenyl (phenyl as a substituent) (recommendation A-13.1).
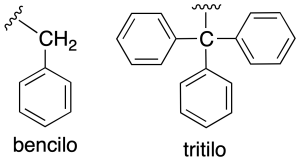
Other radicals very common of them are:
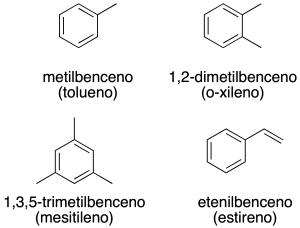
When there are two substituents on the benzene ring may be appointed by the appropriate combination of locators 1,2-, 1,3-, 1,4– or by using the prefixes or– (ortho), m– (goal) and p– (to), respectively (recommendation A-11.3).
We still use some of the common names (recommendation A-11.1), the most common are listed below in parentheses:
Systems polycyclic aromatic can be classified into:
Systems with condensed cycles
share at least two carbon atoms and are named by using the recommendation A-21.1.
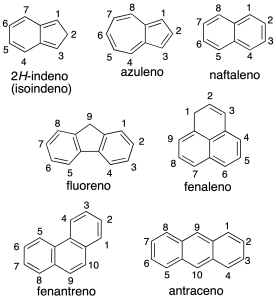
The numbering of the hydrocarbon fused polycyclic is given by the recommendation A-22. In the above figure shows the numbering of some of the compounds. To order numbering system hydrocarbon correctly, the individual rings are drawn in the following way:
We draw two perpendicular axes (dashed lines) and the system polycyclic is oriented in such a way that:
- Present the maximum number of rings on the horizontal axis.
- Present the maximum number of rings in the upper right quadrant.
- If two or more orientations have met the preceding paragraphs, choose the one that has a smaller number of rings in the lower left quadrant.
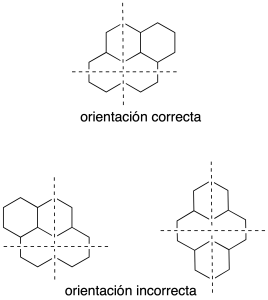
The system is oriented correctly, numbered in the clockwise direction starting at the carbon that is located first in the counterclockwise ring located in the right upper quadrant, if you could choose the one that is farthest to the right.
Atoms that share two or more rings are numbered with the number of the atom that precedes it, followed by the letter (a, b, c, etc). The atoms inside are assigned the highest numbers in a sequence in a clockwise direction whenever possible.
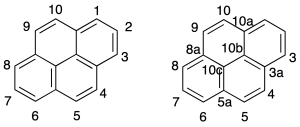
The carbon atoms common to two or more rings the numbers lower.
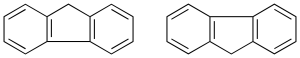
Cycles in the u.s. through a C-C bond simple
They can be equal or different cycles (recommendation A-53), the main structure corresponding to the one with the largest number of links. The secondary structure is numbered with quotation mark ‘.
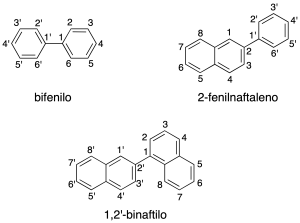
The radicals derived from these hydrocarbons are named with the suffix –yl.
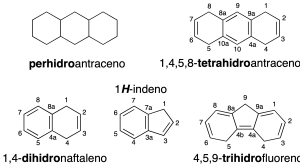
- Arenes partially hydrogenated: Are named as the arene starting, indicating locators hydrogens are added to the structure (dihydro-, trihydro-, tetrahydro-, pentahydro-, …) is used the prefix perhydro– when all the carbons are saturated. If it is only one hydrogen is added is expressed as H– preceded by the locator (recommendations-23.1 and 23.2). In the case of structures with common names as in the following figure, use the separate numbering system.
Return to the page naming mono-functional compounds.