What are haloalkanes?
Haloalkanes (or alkyl halides) are derivatives of hydrocarbons in which one or more of the hydrogens have been replaced by halogens.
Any of the hydrogens in a hydrocarbon can be replaced by halogen. In fact, all the hydrogens in a molecule can be replaced.
For example, in fully fluorinated compounds, they are called fluorohydrocarbons, and are of great industrial interest due to their high thermal stability.
As a general notation for haloalkanes, the expression R—X is often used, where R is any alkyl group and X is any halogen.
Structure and physical properties
To better understand the structure of these compounds, the electronic configuration of the ground state of the halogens must be considered.
| Halógeno | Electron configuration |
| F | 1s2 2s2 2p5 |
| Cl | 1s2 2s2 2p6 3s2 3p5 |
| Br | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5 |
| I | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p64d10 5s2 5p5 |
Halogens are missing a single electron (indicated as rojo in the table) to complete their last shell. Therefore, halogens can be expected to form stable molecules by means of a single covalent or ionic bond with another atom.
On the other hand, halogens have a tendency to attract an electron and are therefore very electronegative atoms (see electronegativity table).
In addition, they have external non-bonding orbitals with unshared electron pairs. Thus, it can be said that the covalent combinations of halogens may be completed as Lewis bases (electron donor).
The structure of methyl fluoride (CH3F) would be:
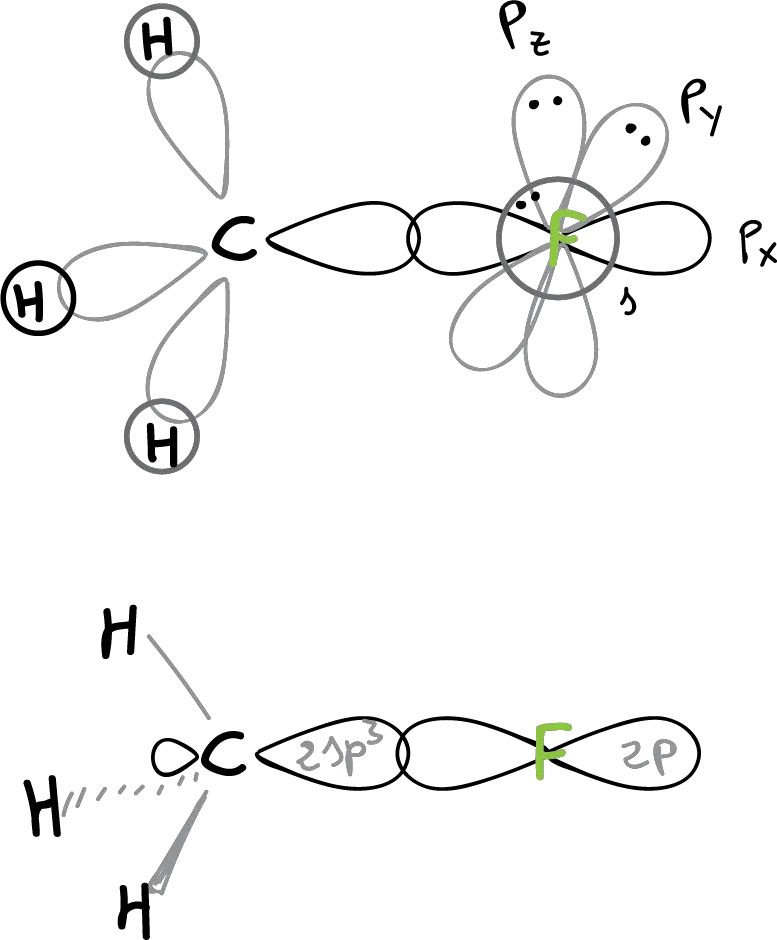
The C—F bonding is realized by overlapping a 2sp3 hybrid orbital of the carbon with a 2p orbital of the fluorine. The angles ∠H-C-H and ∠H-C-F are close to tetrahedral (109º). In this structure, it can be seen how three non-bonding orbitals with 3 unshared electron pairs remain in the fluorine.
Methyl chloride, bromide and iodide are formed by analogous overlaps of a 2sp3 hybrid orbital of carbon with the 3p, 4pand 5p orbitals of Cl, Br and I, respectively.
The strength of the C—X bond decreases as we move down the periodic table group. This is a consequence of the general principle that orbital coating is most effective between orbitals of the same principal quantum level and the effectiveness decreases as the difference between the principal quantum numbers increases.
the bond energies and bond lengths of the four compounds are:
| Bond | Een (Kcal·mol-1) | Distance (Å) |
| C—F | 105.4 | 1.42 |
| C—Cl | 78.5 | 1.77 |
| C—Br | 65.9 | 1.91 |
| C—I | 57.4 | 2.12 |
If we look at Table 2, and taking into account that halogen atoms increase in size as we go down the periodic table. Thus, the C—F bond length (1.42 Å) is intermediate between a single carbon-hydrogen C—H bond (1.1 Å) and a carbon-carbon C-C bond (1.54 Å). All other bond lengths of the remaining halogens are greater than those of the C—C bond.
Although the C—X bonds of alkyl halides are covalent, they have a polar character, because halogens are more electronegative than carbon. The center of gravity of the electron density does not coincide with the nuclear charge center, and this gives rise to a dipole moment.
Nomenclature of haloalkanes
Physical properties
The physical properties of haloalkanes are quite different from those of the corresponding alkanes. This is a consequence of the size of the halogen and the polarity of the C—X bond. These factors affect the bond strength and length, the polarity of the molecule and the boiling point.
Boiling points are generally higher than the corresponding alkanes and increase with the size of the halogen, due to the increase in molecular weight, and chain size.
Haloalkanes are insoluble in water but soluble in organic solvents. Bromine, iodine and polychloroalkanes are denser than water.
Synthesis of haloalkanes
Chemical properties
Of all the reactions that can be carried out with haloalkanes, there are two fundamental ones, which are substitution reactions and elimination reactions.
The displacement of a halide ion by a nucleophile is known as a nucleophilic substitution reaction. Haloalkanes are one of the types of compounds in which a carbon atom is bonded to an atom or group of atoms (leaving group) that can be displaced in a substitution reaction. In these compounds the electronegativity of the leaving group polarizes the bonds, giving rise to a partial positive charge on the carbon atom and another on the nearest hydrogen atoms.
fig-02
A carbon atom with a positive partial charge is an electrophile center. A Lewis base that is also a good nucleophile can react with such an atom by displacing the leaving group.
fig-03
Reactions of haloalkanes
- Reacciones de sustitución nucleófila
- Classification
- Sustitución nucleófila bimolecular
- Reacciones SN2
- Cinética
- Mecanismo y estereoquímica
- Efecto de disolvente
- Reacciones SN2