Thiols[1] have the functional group consisting of a sulfur and a hydrogen SH bonded to a radical (recommendation C-511.1).
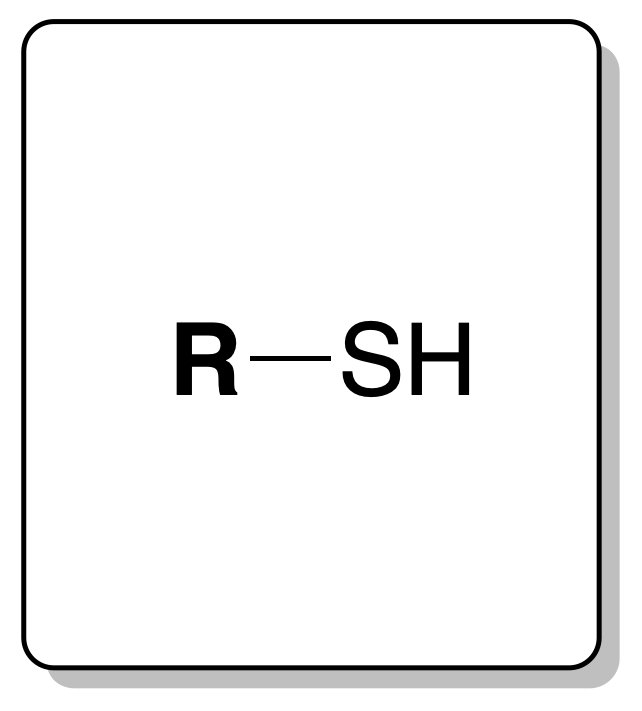
Where R represents an alkyl group or other organic substituent.
Thiols have traditionally been called mercaptans.[1] They are named analogously to the IUPAC form of alcohols (-OH), the –SH group is also called sulfhydryl. For example, the compound CH3SH would be methanethiol, and CH3CH2SH is called ethanethiol.
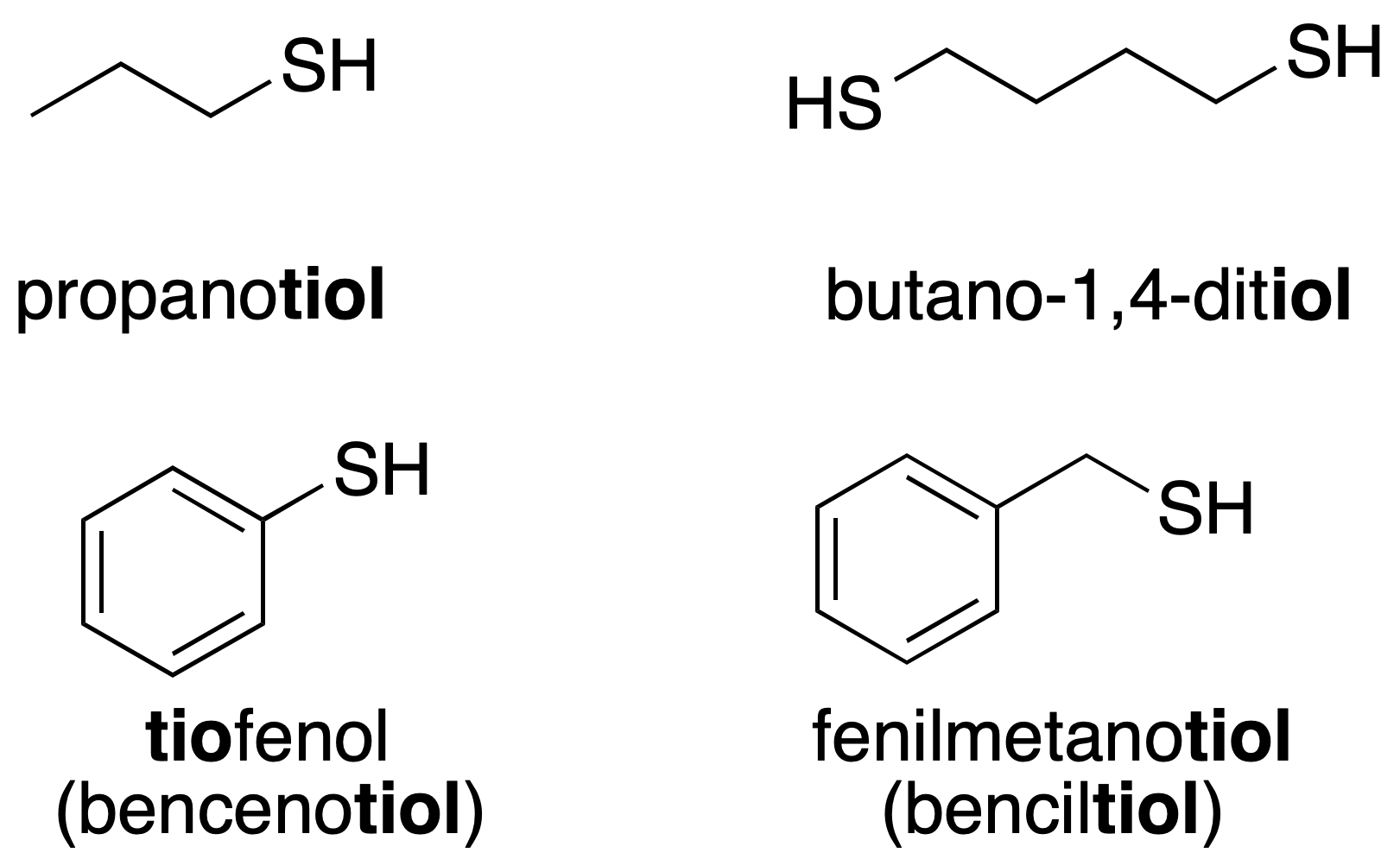
The prefix thio– can also be used to indicate a compound in which an oxygen atom has been replaced by sulfur (recommendation C-511.2).
Steps for name assignment
- The chain that is the longest and contains the –SH is chosen as the main chain.
- We number such a chain so that the carbon attached to –SH has the lowest possible numbers.
- The main chain is named by specifying the number of carbon atoms in that chain with a prefix (ethane– two carbons, propane– three carbons, butane– four carbons; …etc).
- The –SH are identified with their respective numbers separated by commas.
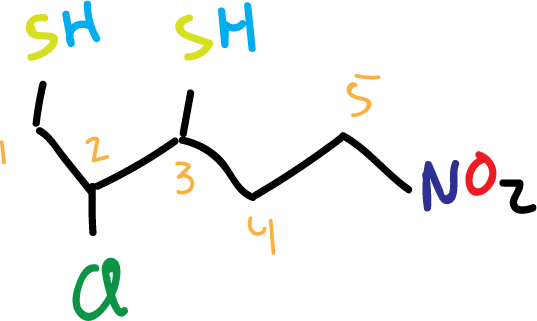
- The termination is placed indicating the number of –SH (-thiol one SH; –dithiol two SH; –trithiol three SH; … etc.).
- The -SH group has priority over double bonds, nitro groups and halogens.
References and notes
[1] The alternative of naming thiols as the radical to which it is attached terminated in mercaptan is discouraged by IUPAC.
Return to the page naming mono-functional compounds.