Objective
To know techniques of magnetic agitation and separation of two immiscible liquids by decantation for the preparation of biodiesel from vegetable oils.
Background
Biofuels are an alternative to fossil fuels as an energy source. In theory, vegetable oils could be used as fuel, but due to their high viscosity, they cannot be used directly.
It is necessary to transform them into other less viscous materials that can be used in vehicle engines. This transformation is carried out by means of a transesterification reaction.
Vegetable oils are compounds belonging to the triglyceride family. By treatment with a base and an alcohol (MeOH or EtOH), an oil is converted into glycerin and methyl or ethyl ester. The transesterification reaction is a catalytic process consisting of two reaction steps.
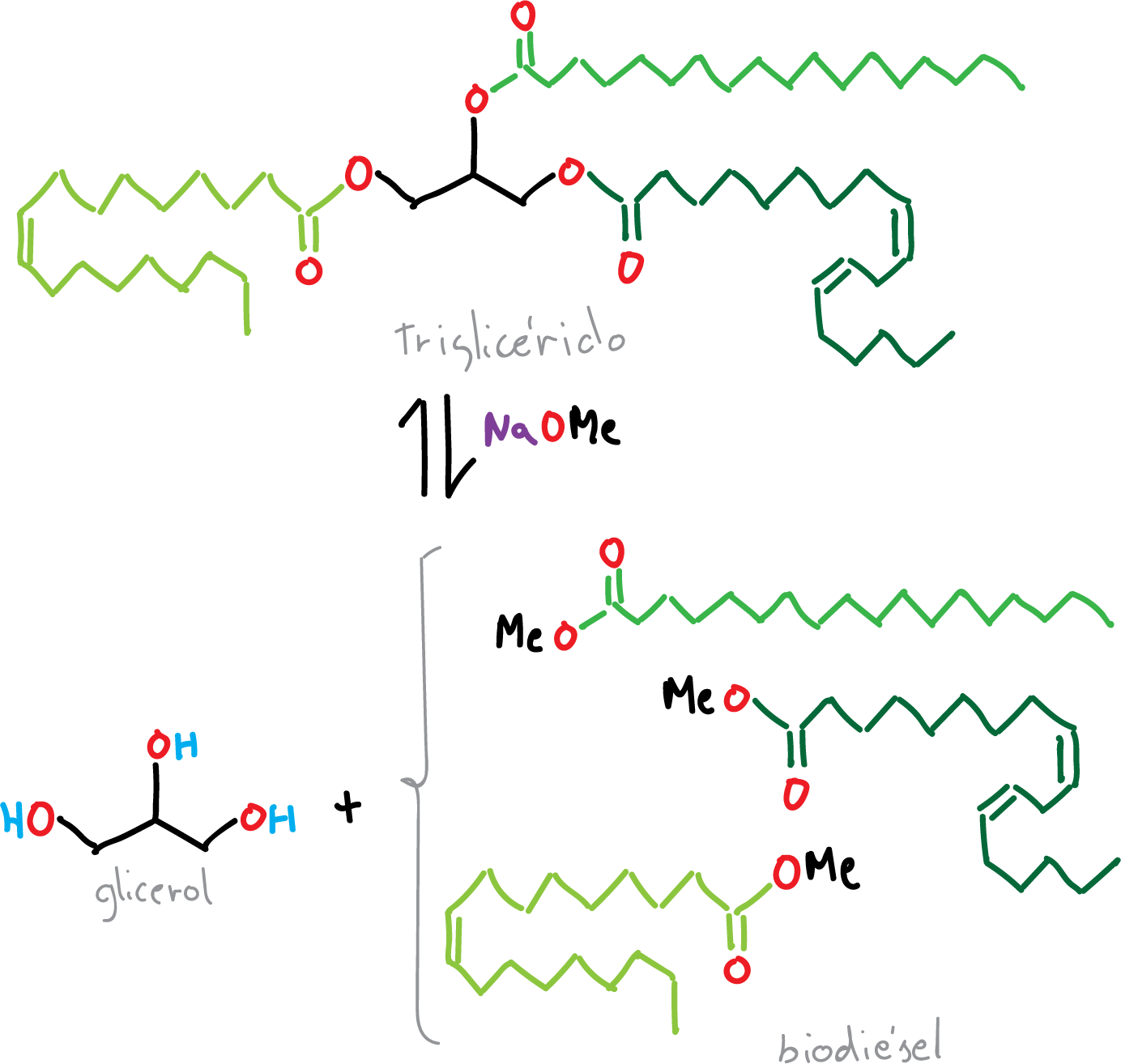
In the first, NaOH reacts with MeOH in a process of formation of acid-base sodium methoxide, a very strong base and water.
In the second step, sodium methoxide acts as a nucleophile by attacking the carbonyl carbon of the vegetable oil, producing a tetrahedral intermediate.
In the final step, the methyl ester is separated from the glycerol. When the experiment is performed with feedstock such as vegetable oil, the reaction proceeds smoothly and gives a good yield of biodiesel. If the crude oil is cooked, the process is more complex due to the presence of free fatty acids from the hydrolysis of triglycerides.
In the basic transesterification process, free fatty acids are deprotonated, leading to the formation of carboxylates that do not react by base-catalyzed esterification.
This not only reduces the reaction yield but also complicates the separation process as long-chain carboxylates give rise to emulsions.
Experimental procedure
In a 100 ml Erlenmeyer flask equipped with a clamp, stir 40 ml of MeOH and 1.3 g of solid NaOH at room temperature to obtain a solution.
In addition, add 130 ml of vegetable oil to a 250 ml round bottom flask. Soybean, sunflower and olive oil can be used for the practice. Heat the flask with the oil to 50 °C using a water bath with magnetic stirring (bain-marie). Add sodium methoxide solution to the oil and shake the mixture for 1 h, maintaining the indicated temperature.
Transfer the contents of the flask to a separating funnel. Let the mixture stand until the two layers separate. The less dense (upper) layer corresponds to biodiesel, the denser (lower) to glycerol. Most of the settling process occurs in the first hour; however, very often the process takes several hours to completely separate the two layers. Decanting can be left overnight.
Use the stopcock of the separating funnel to separate the two layers. If the glycerol is not poured freely, the biodiesel can be removed at the top of the separating funnel with a pipette. The resulting volume is measured with a measuring cylinder and weighed in the same cylinder to determine the density.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| MeOH | 32.04 | -98 | 64.7 | 0.791 |
| MeONa | 54.02 | - | - | 0.970 |
| Glycerol | 92.09 | 20 | 182 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| NaOH |  |
| MeOH |    |
| MeONa |   |
| Glycerol | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| MeOH | OKKJLVBELUTLKV-UHFFFAOYSA-N |
| MeONa | WQDUMFSSJAZKTM-UHFFFAOYSA-N |
| Glycerol | PEDCQBHIVMGVHV-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- D. Bladt, S. Murray, B. Gitch, H. Trout, and C. Liberko, Acid-catalyzed preparation of biodiesel from waste vegetable oil: an experiment for the undergraduate organic chemistry laboratory, Journal of Chemical Education 88 (2011), no. 2, 201–203, DOI: 10.1021/ed9000427
Return to the Organic Synthesis Experiments.
Articles on the website