Objective
The purpose of this practice is to obtain an asymmetric ether of industrial interest from naphthalen-2-ol (β-naphthol) using the Williamson synthesis.
Background
Ethers can be obtained from alcohols by nucleophilic substitution reactions (SN2).
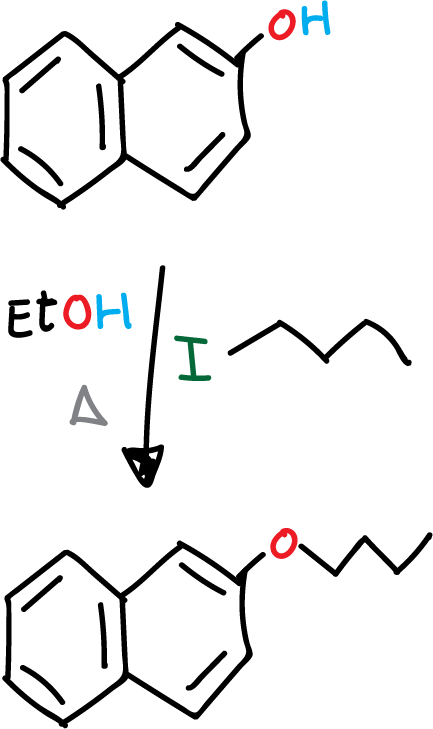
The Williamson synthesis is a process that allows the preparation of a wide range of ethers, both symmetrical and asymmetrical, as long as the alcohol is not hindered, since the structure of each reactant can be easily varied. The reaction proceeds from an alcohol and an alkyl halide in a basic medium.
Reaction mechanism
Ether formation takes place in basic media, the mechanism through which this process takes place involves the following steps:
- Deprotonation of β-naphthol in basic medium.
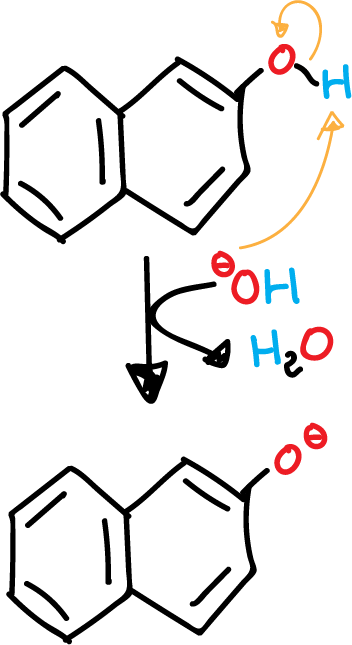
The β-naphthol has a phenol (aromatic alcohol) that can be easily deprotonated. A phenolate (aromatic alkoxide) is obtained, which is more nucleophilic than the initial alcohol.
- Nucleophilic substitution reaction.
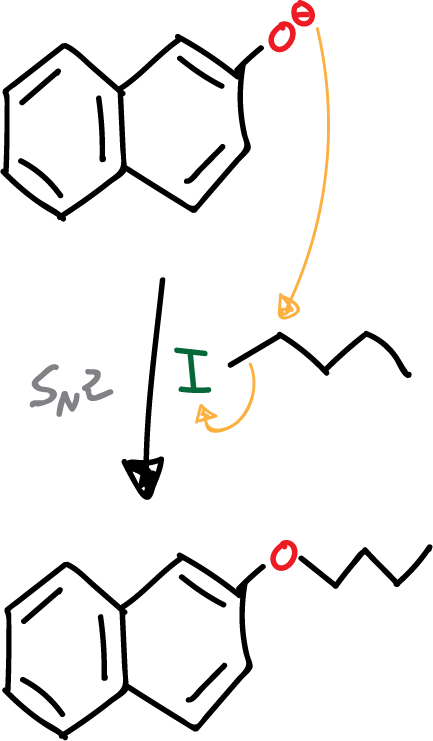
Subsequent to deprotonation, the nucleophilic phenolate is attacked by alkyl iodide. Since we have a good nucleophile and a very good leaving group on a primary carbon, the reaction proceeds via an SN2-type mechanism.
Experimental procedure
Synthesis of 2-butoxynaphthalene
To a 100 mL two-neck flask add 1 g (6.9 mmol) 2-naphthol, 0.56 g (14 mmol) NaOH and EtOH (20 mL). The mixture is heated under reflux until complete dissolution of the reagents.
It is then allowed to cool slightly and 1 mL (8 mmol) of iodobutane is slowly added and the mixture is heated under reflux for 1 hour. After this time, the mixture is allowed to cool and is added to a 250 mL beaker with about 25 g of ice. A white precipitate corresponding to 2-butoxynaphthalene is observed to form.
The contents of the flask can be washed down with cold water. The contents of the beaker are shaken with a glass rod until most of the ice melts. Then filter under vacuum, washing the solid with cold water. If precipitate formation is observed in the filtrate, filter again. The solid is allowed to dry by passing the air stream in the kitasate for a few minutes and then for 24 hours in a desiccator.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| β-Naphthol | 144.17 | 120-122 | 285-286 | 1.280 |
| 2-Butoxynaphthalene | 200.28 | 35-36 | 315.3 | 1.016 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| 1-Iodobutane | 184.02 | -103.5 | 127-133 | 1.617 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| β-Naphthol |   |
| 2-Butoxynaphthalene |  |
| NaOH |  |
| EtOH |  |
| 1-Iodobutane |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| β-Naphthol | JWAZRIHNYRIHIV-UHFFFAOYSA-N |
| 2-Butoxynaphthalene | CDMIQAIIIBPTRK-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| 1-Iodobutane | KMGBZBJJOKUPIA-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Esteb, J. J.; Magers, J. R.; McNulty, L.; Morgan, P.; Wilson, A. M. (2009) A Simple SN2 Reaction for the Undergraduate Organic Laboratory. Journal of Chemical Education, 86 (7), 850−852. DOI: 10.1021/ed086p850
- Khuong, K. S., Agnelli, F., and Parker, M. A. (2023). An updated simple SN2 reaction for the undergraduate organic laboratory. Journal of Chemical Education, 100(1), 376-379. DOI: 10.1021/acs.jchemed.2c00794