Objective
To learn how to prepare a liquid synthetic detergent, through the sulfonation of an aromatic ring.
Background
Detergents are surfactants (consisting of both hydrophobic and hydrophilic fragments), i.e. they greatly reduce the surface tension of water when used at very low concentrations. Detergents have a hydrophilic polar head, such as salts of carboxylic or sulfonic acids, which allows their solubility in water and a carbon chain that gives this part of the molecule a hydrophobic character, thus helping to remove fats and oils mainly from clothes.
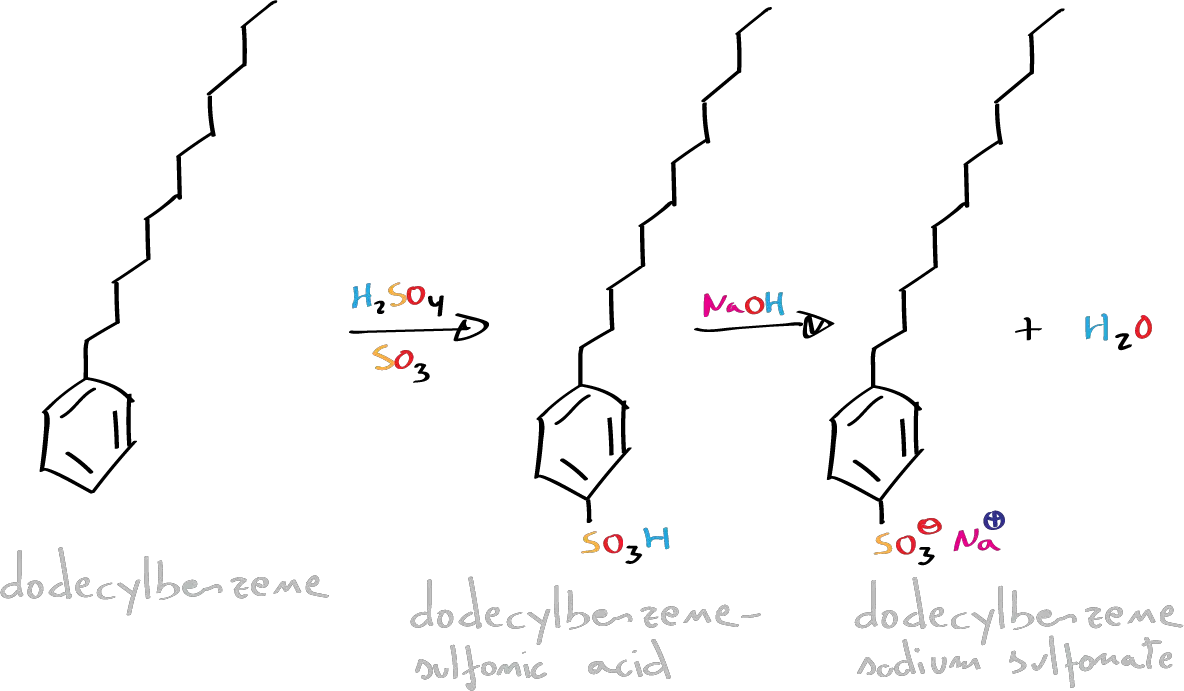
To facilitate the biodegradation of these detergents, linear rather than branched hydrocarbon chains are used in preference to branched ones. Soaps have traditionally been prepared by a saponification reaction of vegetable oils (glycerol esters), resulting in sodium salts of fatty acids.
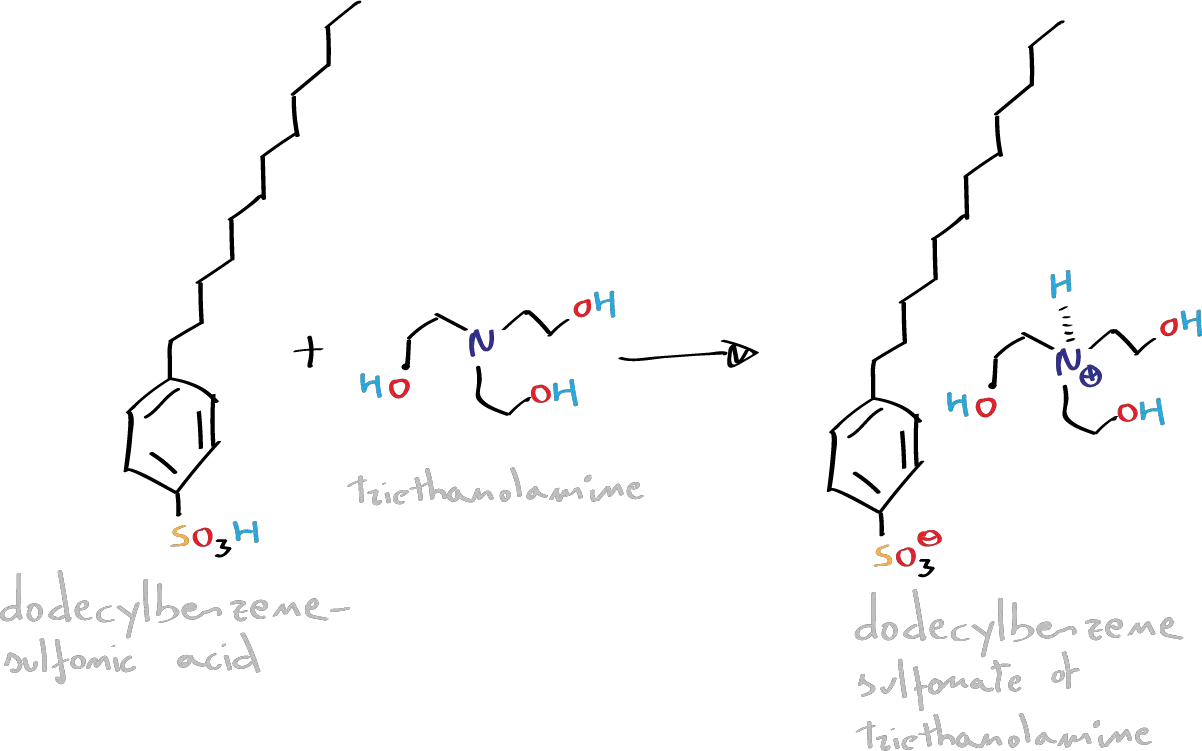
In this experiment, we will proceed to the preparation of a synthetic liquid detergent in the form of ammonium salt of triethanolamine, through a sulfonation reaction of dodecylbenzene (electrophilic aromatic substitution SEAr). Subsequently, with the dodecylbenzenesulfonic acid obtained, a solid detergent will be prepared by treatment with 40 % NaOH.
Experimental procedure
Place 10 g (11.4 ml) of dodecylbenzene in a 100 ml Erlenmeyer flask. Add dropwise for 30 min, stirring constantly 20 g (10 ml) of fuming H2SO4 (great caution in its handling) to the dodecylbenzene, maintaining the temperature between 40-45 ºC with a water/ice bath. Once the addition is finished, the reaction mixture is heated in a water bath at 50 ºC for 5 min, stirring constantly.
To cool the mixture to room temperature and to introduce it in a decanting funnel until it separates in two phases. Once separated, carefully neutralize 2/3 parts of the obtained dodecylbenzenesulfonic acid, adding drop by drop a solution of NaOH at 40 %. The remaining part is neutralized with triethanolamine following the same procedure in order to obtain a liquid detergent.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Sodium dodecylbenzenesulfonate | 348.48 | - | - | - |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Triethanolamine | 149.19 | 17.9-21 | 190-193 | 1.124 |
| Triethanolamine dodecylbenzenesulfonate | 475.68 | - | 264 | 1.200 |
| Dodecylbenzenesulfonic acid | 326.49 | - | 82 | 1.06 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Sodium dodecylbenzenesulfonate |   |
| H2SO4 |  |
| NaOH |  |
| Triethanolamine | Non-hazardous |
| Triethanolamine dodecylbenzenesulfonate |  |
| Dodecylbenzenesulfonic acid |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Sodium dodecylbenzenesulfonate | VQOIVBPFDDLTSX-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Triethanolamine | GSEJCLTVZPLZKY-UHFFFAOYSA-N |
| Triethanolamine dodecylbenzenesulfonate | AVQBNVFOJDNROP-UHFFFAOYSA-N |
| Dodecylbenzenesulfonic acid | WBIQQQGBSDOWNP-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- J. A. Poce-Fatou, A superficial overview of detergency, Journal of Chemical Education 83 (2006), no. 8, 1147, DOI: 10.1021/ed083p1147