What are pyrazoles, triazoles and tetrazoles?
Derivatives of pyrazoles, triazoles and tetrazoles are stable aromatic compounds. Many of them have been used as pharmaceuticals, dyes and pesticides.
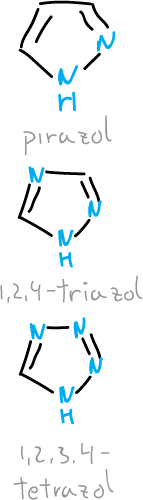
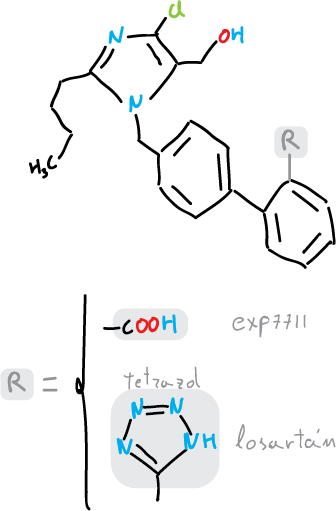
The tetrazoles are relevant in medicinal chemistry. This is because the tetrazole group behaves similar to carboxylic acids. Both systems are planar, have comparable pKa (that of the tetrazol is slightly higher) and in both the electron density is delocalized. When two groups are interchangeable in drugs without changing their biological properties they are called bioisosteres. Compounds can be designed with tetrazoles analogs of amino acids and natural carboxylic acids.
For example, in the case of the hypertensive losartan and the derivative with the corresponding carboxylic acid group (Exp7711):
Ring syntheses can be carried out by both cyclization and cycloaddition reactions.
Synthesis of pyrazole rings
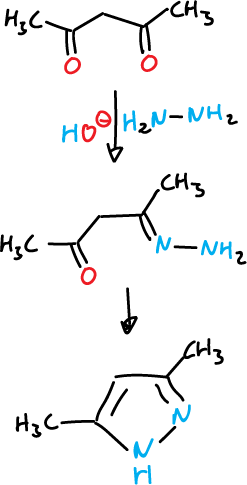
The most general route of synthesis is the reaction of 1,3-dicarbonyl compounds or equivalents with hydrazines.
For example, 3,4-dimethyl-pyrazole is obtained from pentane-2,4-dione and hydrazine.
Another method of obtaining pyrazoles is by reaction of α-haloketones with hydrazines of the XC(S)-NH–NH2 type (where X = SR o NR2).
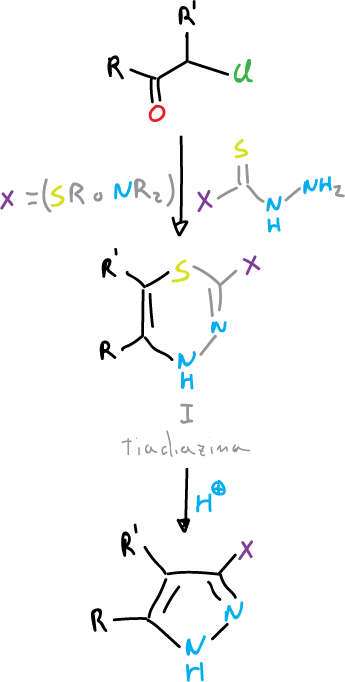
Thiadiazine is produced as an intermediate (I), and this either spontaneously or by acid treatment gives off sulfur to give pyrazoles with good yield.
Another method of pyrazole synthesis is achieved by the cyclization of acetylenic hydrazones.
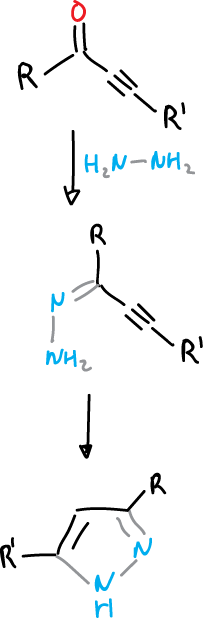
Pyrazole syntheses can also be carried out by electrocylation of unsaturated diazocompounds.
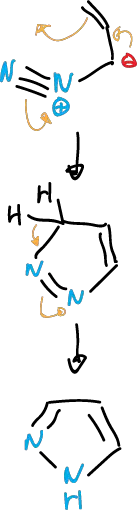
Synthesis of 1,2,4-triazole rings
The most important methods of synthesis of 1,2,4-triazoles are based on the construction and cyclization of structures with skeletons: N—C—N—N and C—N—C—N—N.
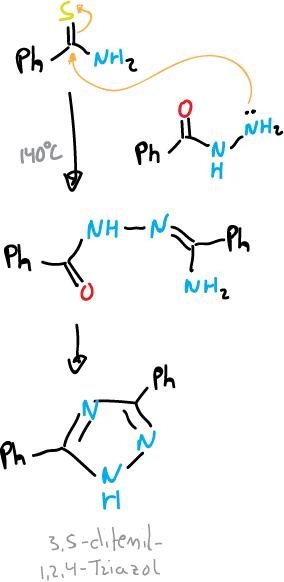
An example of the N—C—N—N type would be the Pellizzari reaction, which consists of the thermal condensation of an acylhydrazine with an amide or a thiamide.
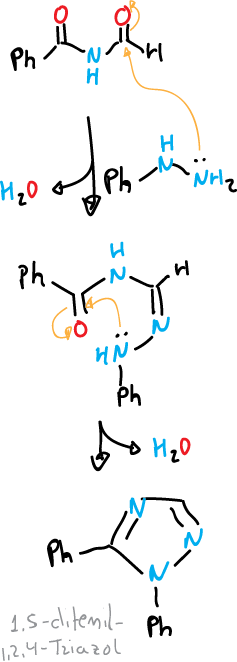
The second type C—N—C—N—N reaction would be represented by the Einhorn-Brunner reaction. This reaction consists of the condensation of a hydrazine or a monosubstituted hydrazine with a diacylamine in the presence of a weak acid. The 1,2,4-triazoles are obtained in good yield.
Synthesis of 1,2,3-triazole rings
The most widely used method of synthesis of 1,2,3-triazoles is the 1,3-dipolar cycloaddition of a variety of organic azides with acetylenes.
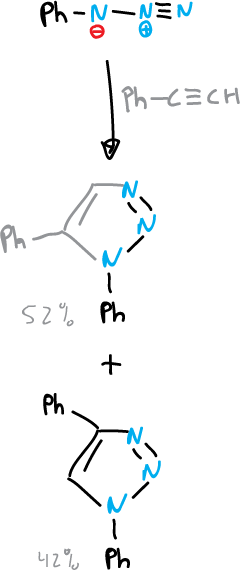
Synthesis of tetrazoles rings
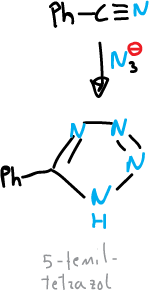
Normally, the synthesis of tetrazoles is carried out from azides, by addition to compounds with multiple carbon-nitrogen bonding.
For example, the synthesis of 5-phenyl-tetrazole from benzonitrile and sodium azide.
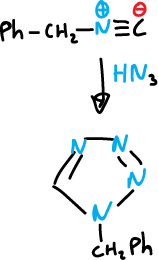
Or, the synthesis from benzyl isocyanide and hydrazoyl acid in the presence of traces of sulfuric acid.
On the other hand, 2,5-disubstituted tetrazoles can be obtained by reaction of amidrazones withnitrous acid.
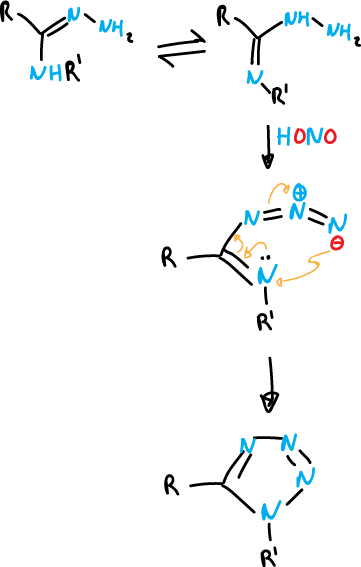
where R is an iminoalkyl group (R—NH—) and R‘ is (H).
Cyclization of the higher charge density imine nitrogen is favored.
Pyrazole, thiazole, tetrazole reactions
The chemistry of these azoles increasingly differs from that of pyrrole as the number of nitrogen atoms increases.
Electrophilic substitution reactions on carbon are rare. This is due to the smaller number of carbon atoms and the fact that electrophiles preferentially attack nitrogen.
Electrophilic substitution on a ring nitrogen bonded with multiple bonding
In azoles containing two ring nitrogen atoms, the imine nitrogen (=N) is easily attacked by electrophiles and, generally, the attack is followed by the loss of the proton of the other nitrogen (N-H).
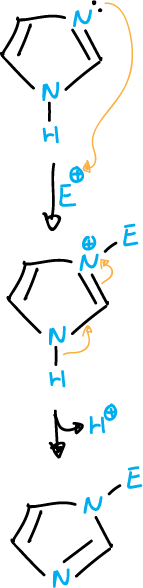
As the electrophile reagent attacks the multiply bonded nitrogen atom (highlighted with arrows ⇒ in the schematic).
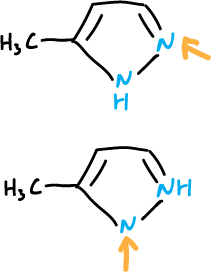
The orientation of the substitution is related to the structure of the starting tautomer.
However, it must also be considered that regardless of the percentages of the tautomeric forms, if one of them reacts faster, it can control the reaction.
The pyrazoles are readily alkylated with iodomethane (CH3I) or methyl sulfate (CH3SO4). Generally, asymmetric compounds are a mixture of products whose composition depends on the conditions.
The pyrazole can be brominated at the C4 position under mild conditions.
El 4-nitro pyrazole se puede preparar también en forma directa a partir del pyrazole.
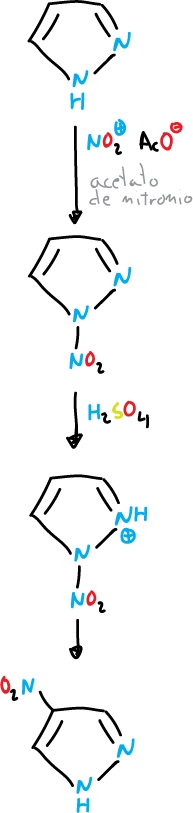
1-nitro pyrazole is an effective nitrating agent for aromatic hydrocarbons in the presence of acids.
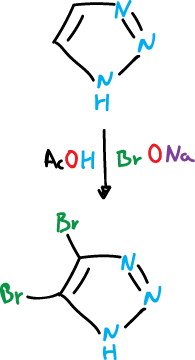
1,2,3-triazole, by treatment with sodium hypobromite (BrONa) in acetic acid (AcOH), is converted to the 4,5-bromoderivative.
The 1,2,4-triazole is chlorinated at C3 carbon atom.
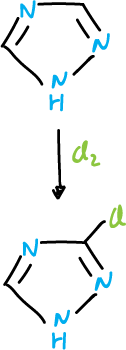
From N-substituted azoles and alkylating agents, quaternary salts can be formed, for example, 1-methyl-1,2,4-triazole is successively methylated in N4 and N2 with an excess of trimethyloxonium tetrafluoroborate.
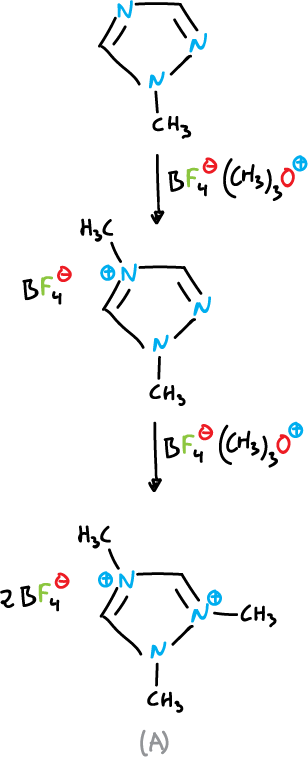
Diquaternary salt (A) can be isolated as a crystalline solid.
Nucleophilic substitution
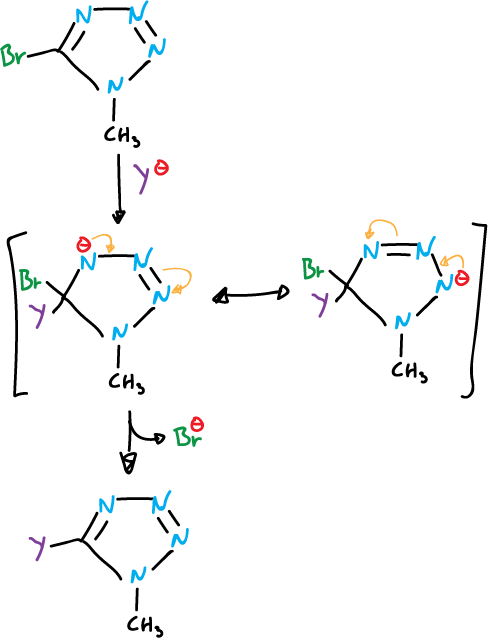
In several azoles nucleophilic substitution reactions occur with substituents on the carbon, which are good 5-bromo and 5-chloro-tetrazoles 1-substituted leaving groups.
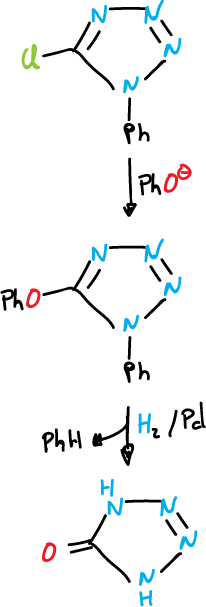
The base-catalyzed reaction of 5-chloro-1-phenyl tetrazole with phenols leads to the formation of ethers.
Ethers can be reductively cleaved by treatment with hydrogen and palladium (H2/Pd). This is a useful procedure for deoxygenating phenols.
The facility to displace halides from these azoles increases with the presence of additional attracting electron groups on the carbon atoms of the ring or by quaternization of nitrogen.
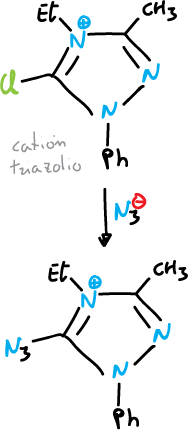
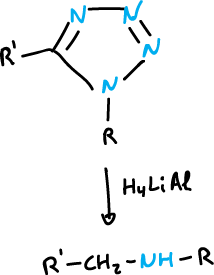
Ring opening
Some 1,2,3-triazoles and tetrazoles are broken down by heating.
Ring-chain tautomerism is common for 1,5-disubstituted tetrazoles.
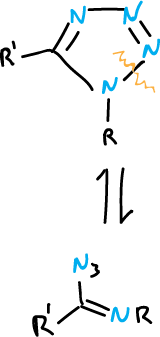
Also, it occurs with more difficulty in the 1,2,3-triazoles substituted at the N1 position.
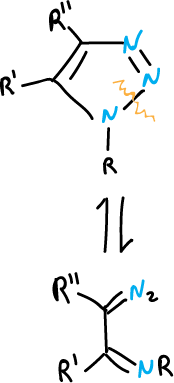
Ring opening is facilitated when there are electron attracting groups at the N1 position. Open-chain tautomers can be reversibly cyclized, or also transpose or undergo other reactions in which they lose nitrogen. This occurs depending on the nature of the substituents and the reaction conditions.
An example of the first case is the Dimroth transposition, which take place when tetrazoles or 1,2,3-triazoles with an amino substituent at the C5 position are heated.
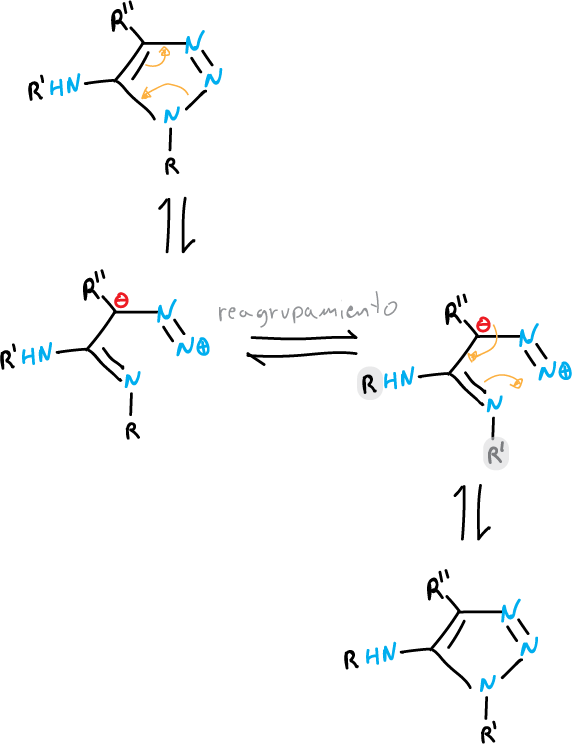
Theposition of the equilibrium is influenced by the nature of the substituents and the pH of the solvent.
The action of heat (Δ) or light (hν) can cause the expulsion of nitrogen from tetrazoles and 1,2,3-triazoles.
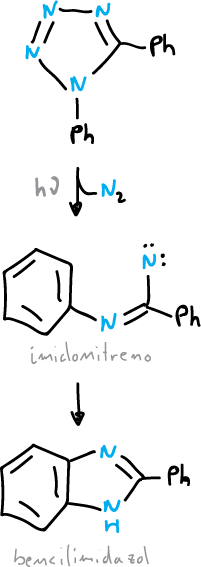
An important application of this reaction is the use of tetrazole-5-diazonium chloride as a source of carbon atoms.
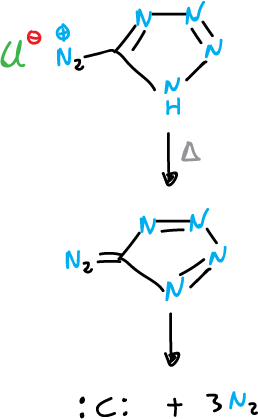
The reaction is carried out in the presence of reagents capable of intercepting the monocyclic carbon produced.