Substituted benzenes
As we can see, the variety of substituents that can be introduced directly on the aromatic ring is not very wide. However, it is possible to transform them into a wide variety of other functions by means of relatively simple reactions, for example:
Halogenation of alkylbenzenes at the benzyl position
Benzyl hydrogens have a special reactivity. Therefore, one of these reactions is the substitution by halogens by means of a radical mechanism. Therefore, only chlorination and bromination have synthetic applications. Thus, Cl2 or Br2 can be used when heated or illuminated with radiation of a suitable wavelength. Alternatively, N-chlorosuccinimide (NCS) or N-bromosuccinimide (NBS) can be used as halogen sources.
Under these conditions, no reaction takes place on the aromatic ring. Therefore, the key to the success of this reaction lies in the stability of the benzyl group (reaction intermediate).
On the other hand, benzyl halides are useful compounds used in SN2 reactions or in the preparation of alkenylbenzenes.
fig1
fig2
Oxidation of alkylbenzenes
Benzene derivatives with an alkyl chain with at least one hydrogen in the benzyl position can be oxidized to give a carboxyl group, regardless of the length or branching of the alkyl group. K2Cr2O7 or K2MnO4, in hot acidic media, is usually employed as oxidant.
fig3
Aromatic aldehyde and ketone reduction (Clemmensen and Wolff-Kishner)
Aromatic ketones can be reduced to the corresponding saturated compound by two procedures:
1) Clemmensen reduction: treatment with zinc amalgam and hot HCl.
2) Wolff-Kishner reduction: treatment with hydrazine in basic medium in a high-boiling solvent.
fig4
Under these conditions neither the double bonds, C=C, nor the triple bonds, C≡C, nor the COOH group are reduced. The choice of one method or the other is going to depend on the compatibility of the rest of the functions with the acidic conditions of the Clemmensen reaction or basic conditions of the Wolff-Kishner reaction.
These reactions, together with Friedel-Crafts acylation, are an alternative to Friedel-Crafts alkylation when trying to introduce an alkyl chain from a primary alkyl halide, since as seen previously, transpositions occur when trying to introduce long aliphatic chains by direct alkylation, see Friedel-Crafts alkylation.
fig5
Oxidation of benzyl alcohols
The hydroxyl groups in the benzyl position are oxidized to carbonyl groups with mild oxidants such as MnO2 (manganese dioxide).
fig6
Reduction of nitro groups
Nitro groups attached to benzene rings can be easily reduced to amine by several procedures. The most commonly employed in aromatic nitro compounds are:
- Catalytic hydrogenation in the presence of Pt as catalyst.
- Treatment with metals (Fe or Sn) and hydrochloric acid. With hydrochloric acid, the chlorohydrate of the corresponding amine is obtained.
- Treatment with SnCl2 and hydrochloric acid.
fig7
Hydrolysis of -SO3H groups
The sulfonation reaction in benzene is reversible, so that by heating sulfonic acid with an aqueous solution of H2SO4 or H3PO4 it is possible to obtain benzene again. It is synthetically useful for directing other electrophilic aromatic substitution reactions to positions that activate the SO3H group and then eliminate it.
fig8
Conversion of haloalkanes to organometallic compounds
Aryl halides treated with metals such as Li or Mg give organometallic compounds, analogous to the way alkyl halides react. These compounds react similarly to aliphatic organometallic compounds.
fig9
Arylamines
Electrophilic aromatic substitution of arylamines
They are very reactive substances in electrophilic aromatic substitution, since the -NH2, -NHR and -NR1R2 groups are strongly activating and ortho- and para– directing, and polysubstitution products often occur. For aniline it is possible to perform direct iodination by treating with I2 and in the presence of a solution of sodium bisulfite, NaHSO4. With Br2 or Cl2polysubstitution products can be formed.
Nitration, sulfonation, alkylation or acylation reactions cannot be carried out directly because they either react with the acid used as catalyst or they oxidize or polymerize as with nitric and sulfuric acids, respectively. To avoid these problems, the -NH2 group is converted to an amide by an acylation reaction.
fig9
Acylation of arylamines
The -NH2 group can be converted to an amide by treatment with an acyl chloride or anhydride. It is not necessary to add a base, since the basicity of the -NH2 group itself catalyzes the reaction. Generally, acetyl chloride or acetic anhydride is used. The amine is regenerated by treatment with hot NaOH solution or hot hydrochloric acid. In this case the chlorohydrate is isolated from the amine.
Another characteristic of the group that is generated is its activating character, although less than that of the free amino, due to the fact that charge density is removed from the benzene ring.
fig9
Synthesis and reactivity of diazonium salts
Diazonium salts in aromatic compounds are of great interest in organic synthesis, since a wide variety of derivatives can be prepared from them.
They are obtained by treatment of an amine with sodium nitrite in an acid medium at low temperature, since they decompose losing nitrogen relatively easily if the temperature is raised.
Diazonium salts may undergo the following reactions:
1. Reduction with hypophosphorous acid to give the corresponding arene.
2. Acid-catalyzed hydrolysis to yield phenols.
3. Substitution reactions:
- Obtaining aryl fluorides by Schiemann reaction:
- Sandmmeyer-type reactions: these are a set of reactions where the diazonium salt is displaced by a nucleophile such as Cl⊖, Br⊖, I⊖, CN⊖ or R-SH, respectively. The -CN groups are precursors of carboxylic acids (-COOH) since hydrolysis in acidic media results in the transformation -CN → -COOH. In many cases, catalysis with Cu(I) is required, so the copper salts of the anions are used. For thiols or iodide, the presence of the catalyst is not necessary. This reaction is important because products are obtained which cannot be produced by direct electrophilic aromatic substitution of the corresponding arene.
- An alternative for the preparation of arylnitriles starts from aryl halides, which with CuCN give the desired product (Rosenmund-von Braum reaction).
4. Azocopulation: the reaction of diazonium salts with activated arenes in neutral or slightly acidic media leads to the formation of azo-compounds (-N=N- azo group). In benzene rings the substitution occurs preferentially on the para-positions of the activating group. It is a method widely used in the synthesis of dyes.
Phenols
Acid-base reactions of phenols
Phenols react with bases to give phenoxides, which are excellent nucleophiles.
fig12
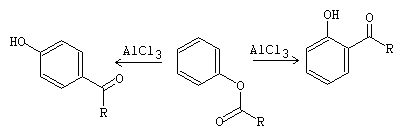
Phenol acylation and Fries transposition reactions
The acylation of –OH occurs in a basic medium and the transposition in an acid medium. Thus, phenol esters can be obtained using acid anhydrides and AlCl3 in the presence of a base such as pyridine. Generally, acetic anhydride is used.
fig13
fig14
The acyl groups dampen the strongly activating character of the –OH group of the phenol, because the electron donating effect of the hydroxyl group is attenuated. Therefore, this is because they produce a decrease of the charge density in the aromatic ring.
Thus, the C-acylation reaction of phenols occurs under thermodynamically controlled conditions. Because the product obtained is more stable. However, the O-acylation product (esterification) occurs under kinetic control conditions.
On the other hand, the aryl esters, resulting from the O-acylation reaction, are transformed with AlCl3 into the corresponding arylketones. This reaction is known as Fries transposition.[1]
fig15
Oxidation reactions of phenols
Phenols are more easily oxidized than alcohols. Many reagents can be used, in particular Ag2O or Na2Cr2O7. In addition, of particular relevance, especially in biological redox systems, are the oxidations of 1,2- and 1,4-diphenols which generate benzoquinones.
fig16
Electrophilic aromatic substitution reactions of phenols
Phenols are very good substrates for the electrophilic aromatic substitution reaction. And as discussed above, the OH group is strongly activating and ortho- and para-directing.
Therefore, due to the higher reactivity of phenols, polysubstitution products are usually obtained. Thus, the conditions of electrophilic aromatic substitution reactions are milder than those of the benzene substitution reaction.
Carboxylation of phenols (Kolbe-Schmitt reaction)
The Kolbe-Schmitt reaction is a phenol transformation reaction, promoted by bases, leading to the formation of salicylic acid and derivatives.
fig17
Summary
The different electrophilic aromatic substitution reactions seen in the previous sections for substituted aromatic compounds can be summarized in the following table for benzene, phenol and aniline derivatives.
| Reaction | Benzene | Phenol | Aniline | |
| Halogenation | X2 / FeX3 | X2 without catalyst | X2 without catalyst < (I2 / HCO32-) | |
| Polysubstitution products | ||||
| Nitration | HNO3 / H2SO4 | HNO3 / H2O | — | |
| Low yield due to oxidations, mixed ortho- and para- | — | |||
| Sulfonation | fuming H2SO4 | H2SO4 conc. | — | |
| Majority ortho- (kinetic control) | — | |||
| Alkylation | R-Cl / AlCl3 | R-OH in acid medium or R-Cl / AlCl3 | — | |
| Acylation | RCOCl / AlCl3 | RCOCl / AlCl3 | CH3COCl | |
References and notes
[1] a) K. Fries, G. Fink, Chem. Ber. 41, 4271 (1908). b) K. Fries, W. Pfaffendorf, Chem. Ber. 43, 212 (1910).