What is reflux?
The vast majority of chemical reactions take place in solution. The vast majority of organic solvents are volatile (VOC) and many are flammable, so if a reaction is warmed in an open vessel, the solvent evaporates shortly thereafter, making contact with the heat source and causing a consequent danger of fire. To heat a reaction safely in the necessary time, a technique called reflux is used (see Figure).
Assembly scheme
A round-bottomed flask with a ground glass joint is placed on a heat source (hot plate or heating mantle). The flask is fixed to the metal rod with a clamp and clamp holder.
Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better grip.
Optionally, when the reaction conditions require it, a drying tube to hold a solid desiccant wil be used which prevents moisture from entering the reaction.
The solution with the reagents is placed in the round-bottom flask and a stir bar or a boiling chip is also added. Finally, the water inlets and outlet of the reflux condenser are connected to the water circuit.
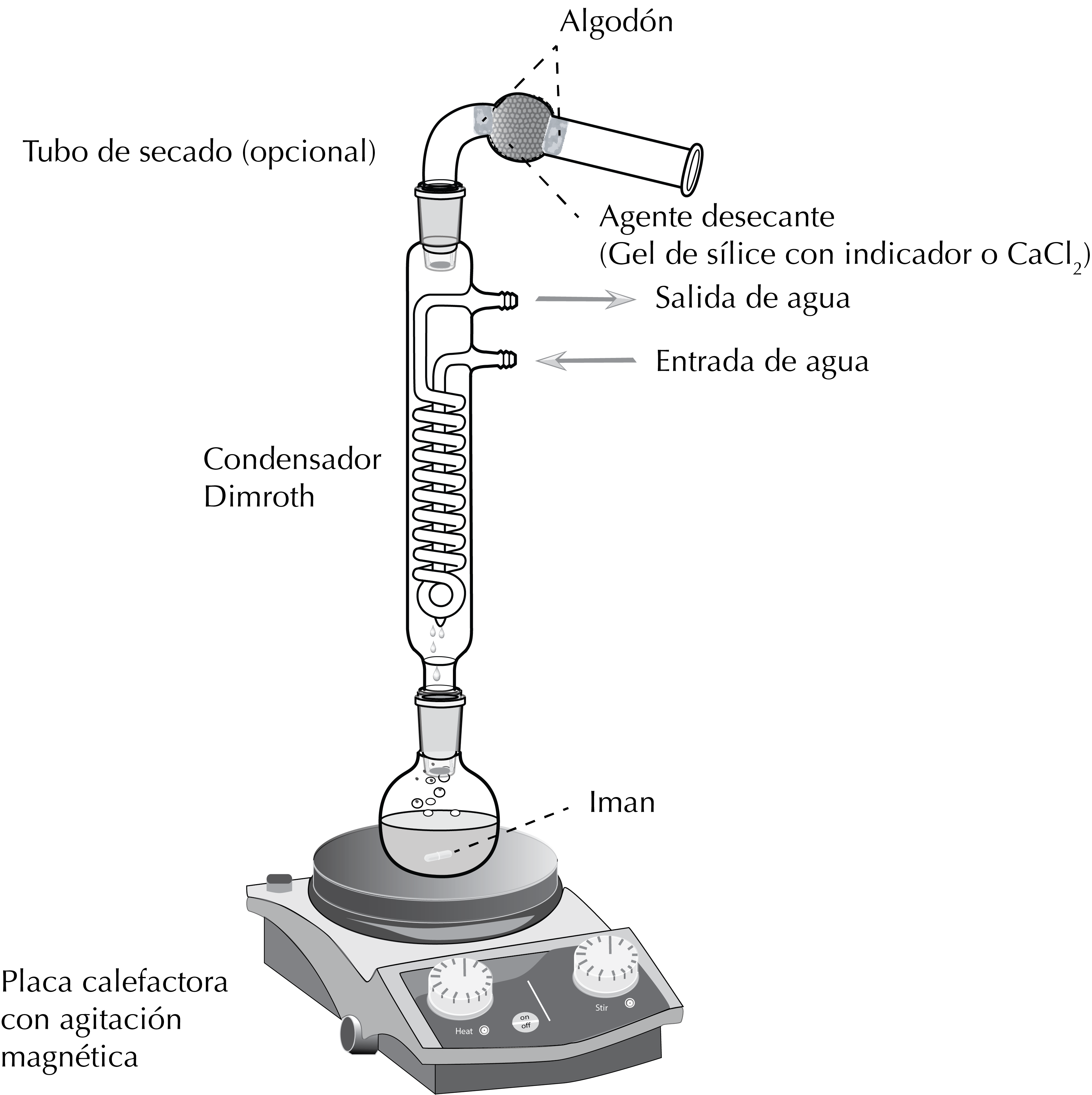
When the solvent begins to evaporate, the vapours ascend to the condenser, where they cool, condense and fall back by gravity to the flask, thus achieving two effects:
- The volume of the solution does not vary.
- The reaction temperature is kept constant, corresponding to the boiling point of the solvent at the pressure at which the experiment is performed.
Thus, the reaction can be heated safely for long periods of time. The boiling should be homogeneous to avoid splashing; for this, magnetic stirring is used.
When the reaction is finished, do not remove the condenser until the reflux has ceased.
Choice of solvent
A critical aspect when carrying out a reflux reaction is the choice of solvent. Depending on the type of reaction, the reagents used, and the temperature at which the reaction is carried out, different solvents can be used.
Sometimes one of the reagents, due to its properties, has a dual role, acting also as a solvent. More commonly, however, a solvent must be chosen, or even mixtures are used. In reflux reactions, the selection of the solvent is critical, since it must meet certain conditions and have characteristics such as:
- Adapting their properties to the type of reaction (polarity, boiling point, protic or non-protic character).
- Dissolving the reactants and products at the boiling point.
- Being inert to reagents and products at boiling point.
- Being easy to remove once the reaction is finished.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2