Tabla de contenidos
¿Qué es el remdesivir?
Remdesivir es un retroviral que ha sido desarrollado por Gilead Sciences como tratamiento para el virus del Ebola y el de Marburg. Seguidamente esta compañía ha reportado su actividad antiviral frenta a corona-virus.
 |
| Estructura 3D |
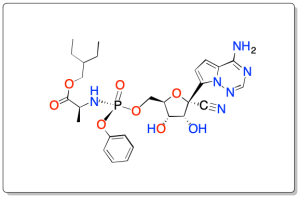
La estructura es de un derivado ciclado de la α-D-ribosa-5-fosfato con dos sustituyentes: 4-amino-pirrolotriazina y ciano en el carbono-2, sustituida en el fosfato con un grupo 2-etilbutil-2-aminopropanoato como se muestra en la figura.
Síntesis
Gilead Science ha patentado un método de síntesis convergente para el Remdesivir a partir de derivados de la ribosa.
Se preparan tres intermedios (I, II y III) que se utilizarán en los pasos finales de la síntesis.
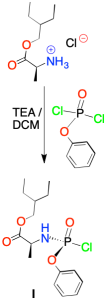
En un primer paso, el intermedio I se prepara a partir de L-alanina y fenildiclorofosfato en presencia de trietilamina (TEA) y diclorometano (DCM).
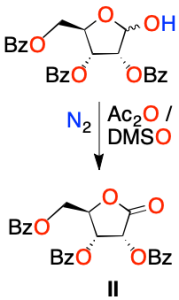
Alternativamente, se oxida la ribosa protegida con tres bencilos, en concreto el grupo –OH hasta carbonilo >C=O. Para ello, se emplea anhídrido acético (Ac2O) en dimetilsulfóxido (DMSO), para formar la lactona II.
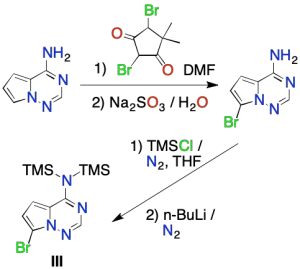
Además, para preparar el intermedio III, primero se broma la 4-amino-pirrolotriazina. Después, se protege el grupo amino (-NH2) con cloruro de trimetilsililo (TMSCl). Una vez protegido el grupo amino, se reemplaza el Br por Li usando n-butil litio para producir el intermedio III.
Una vez obtenidos los tres intermedios, se procede a ensamblarlos. El intermedio II se agrega gota a gota a una disolución que contiene el intermedio III.
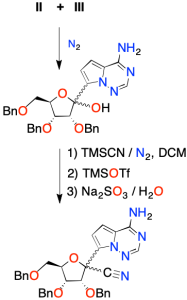
Después de inactivar la reacción en una solución acuosa débilmente ácida, se obtiene una mezcla racémica.
Luego, se hace reaccionar con un exceso de cianuro de trimetilsililo (TMSCN) en diclorometano (DCM). Se añade triflato de trimetilsililo (TMSOTf) y al tratarlo con Na2SO3 en agua se obtiene un intermedio con el grupo nitrilo (-C≡N).
El grupo protector, bencilo, se elimina con tricloruro de boro(BCl3) en diclorometano (DCM).
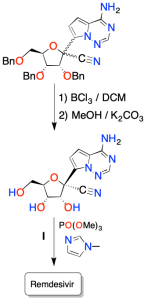
Los isómeros se separaron mediante HPLC de fase inversa. Y el compuesto ópticamente puro y el intermedio I se hacen reaccionar con fosfato de trimetilo y metilimidazol para obtener una mezcla diastereomérica de Remdesivir. Al final, se puede obtener Remdesivir ópticamente puro a través de métodos de resolución quiral.