Objective
To learn how different organic compounds can be isolated according to their acid-base properties, which change their solubility in organic and aqueous solvents, using the liquid-liquid extraction technique.
 |
 |
| 3D structure | 3D structure |
 |
 |
| 3D structure | 3D structure |
Background
Liquid-liquid extraction is one of the most common basic operations in the organic chemistry laboratory, since many reactions involve the use of this technique for product isolation.
Carboxylic acids can react with bases such as sodium hydroxide, producing a proton and forming the corresponding water-soluble anions (carboxylates).
On the other hand, amines in acid media produce water-soluble ions generated by protonation, such as ammonium salts.
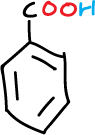
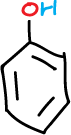
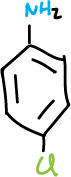
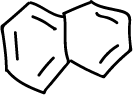
A series of experiments have been designed involving the separation of various components of a mixture consisting of simple organic compounds such as: naphthalene, p-chloroaniline, phenol and benzoic acid, to be dissolved in an organic solvent such as methylene chloride CH2Cl2, depending on its acidic or basic character.
Separation of 2 components of a mixture
Separation of 3 components of a mixture
Separation of 4 components of a mixture
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2