They are characterized by a bond migrating along a p-system to another position in the molecule. Thus, the nomenclature proposed by Woodward-Hoffmann for sigmatropic transposition reactions uses two numbers in square brackets indicating the positions to which the ends of the s-bond migrate.
Suppose the transposition of an R group, where the C-R bond migrates along a p-system, which in this case is the simplest possible since it is a p orbital, to another position where it gives rise to a product that is degenerate with the reactants if the substituents were the same.

In this reaction we see that the bond s migrates to another new position. If we look at the two ends of the migrating bond, one of them remains attached to R and therefore does not move, i.e. if it was in position 1 of that R group, it is still in position 1 of that other R group. However, the other end moves from position 1 to position 2. Therefore, those numbers in square brackets denote to which positions the bond ends move. The 1 means that one end of the bond remains attached to the bond to which it was originally attached and the 2 means that the other end moves to position 2.
In this other example, the thicker link is the one that migrates from one position to another, which would be a transposition [1,3].
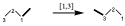
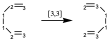
In the following (Cope rearrangement), the two ends of the migrating bond change position and will be a transposition [3,3]. This reaction will be degenerate if there are no substituents.
There are in general transpositions of all types [m,n] where m and n can be any integer.