What is steam distillation?
The vapor distillation is a technique applied in the separation of a substance from a mixture. It takes advantage of the low boiling point property of immiscible mixtures. It is particularly suitable for separating temperature-sensitive organic molecules from a non-volatile contaminant in water-immiscible substances. It has the disadvantage of having very high boiling points, which decompose upon distillation.
Also, this technique is often used to purify substances contaminated by large amounts of resinous impurities, to separate high boiling solvents from solids that do not carry over.
In steam distillation, a steam (usually water vapor) is passed through to entrain the substance.
The immiscible mixture is heated to boiling, which causes distillation of both water and volatile organic compounds. This means that the gaseous mixture travels upward to a condenser, which then condenses the vapor to liquid so that it can be collected.
Unlike simple distillation, it uses a reservoir to replenish the water in the heated mixture throughout the process. The immiscible organic component is slowly distilled along with the water, while the non-volatile component remains in the heated mixture. Once the organic component is distilled, it can be separated from the water by liquid-liquid extraction.
Depending on the way steam is generated, different devices are used and two types of steam distillation are distinguished, depending on whether the steam source is external or internal.
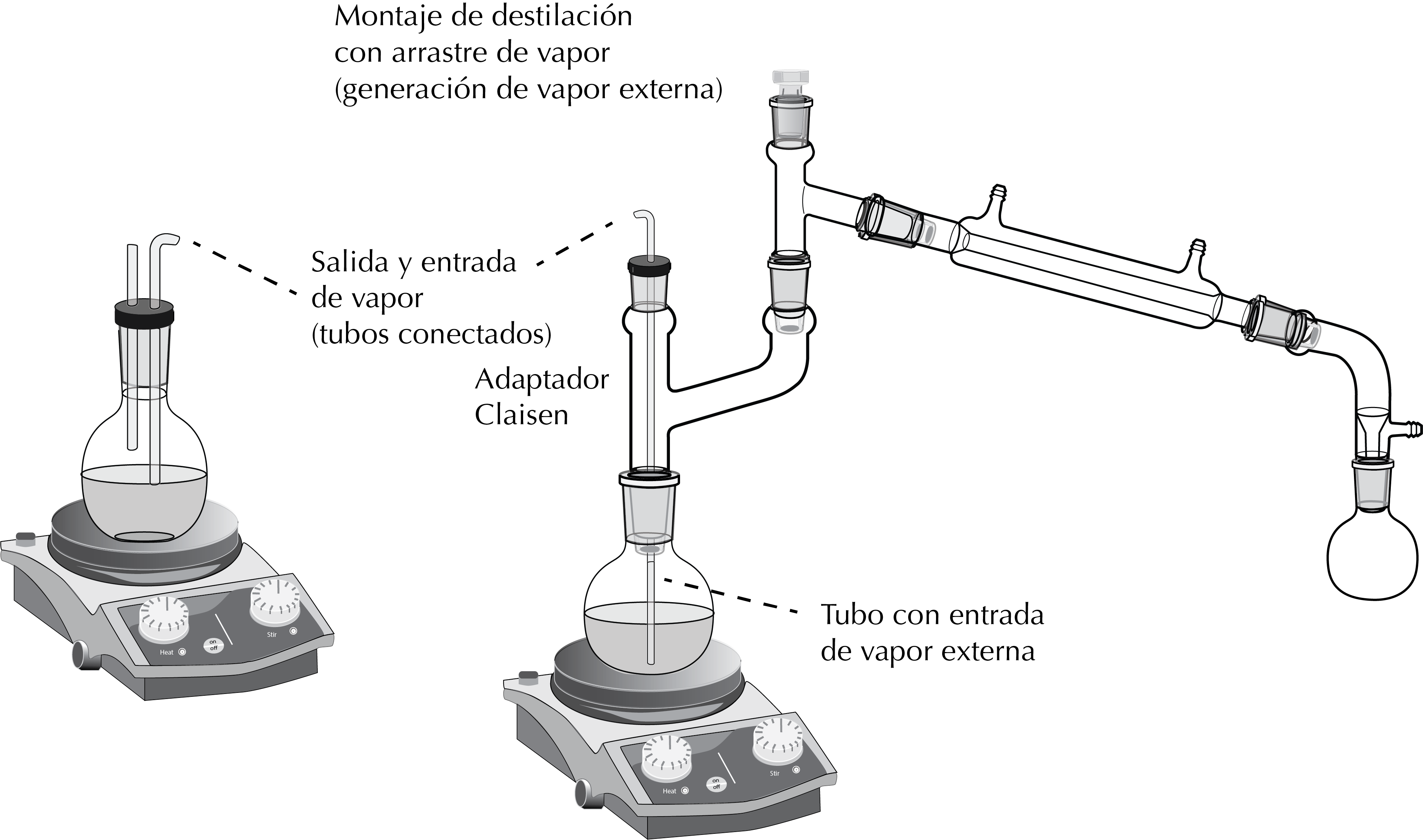
Distillation with external steam source
It is performed with a setup (with the corresponding support) as shown in the Figure, and includes a Claisen adapter. It is similar to that of a reaction with gaseous reactants, except that in this case the gas bubbled over the product solution is water vapor obtained in an auxiliary setup.
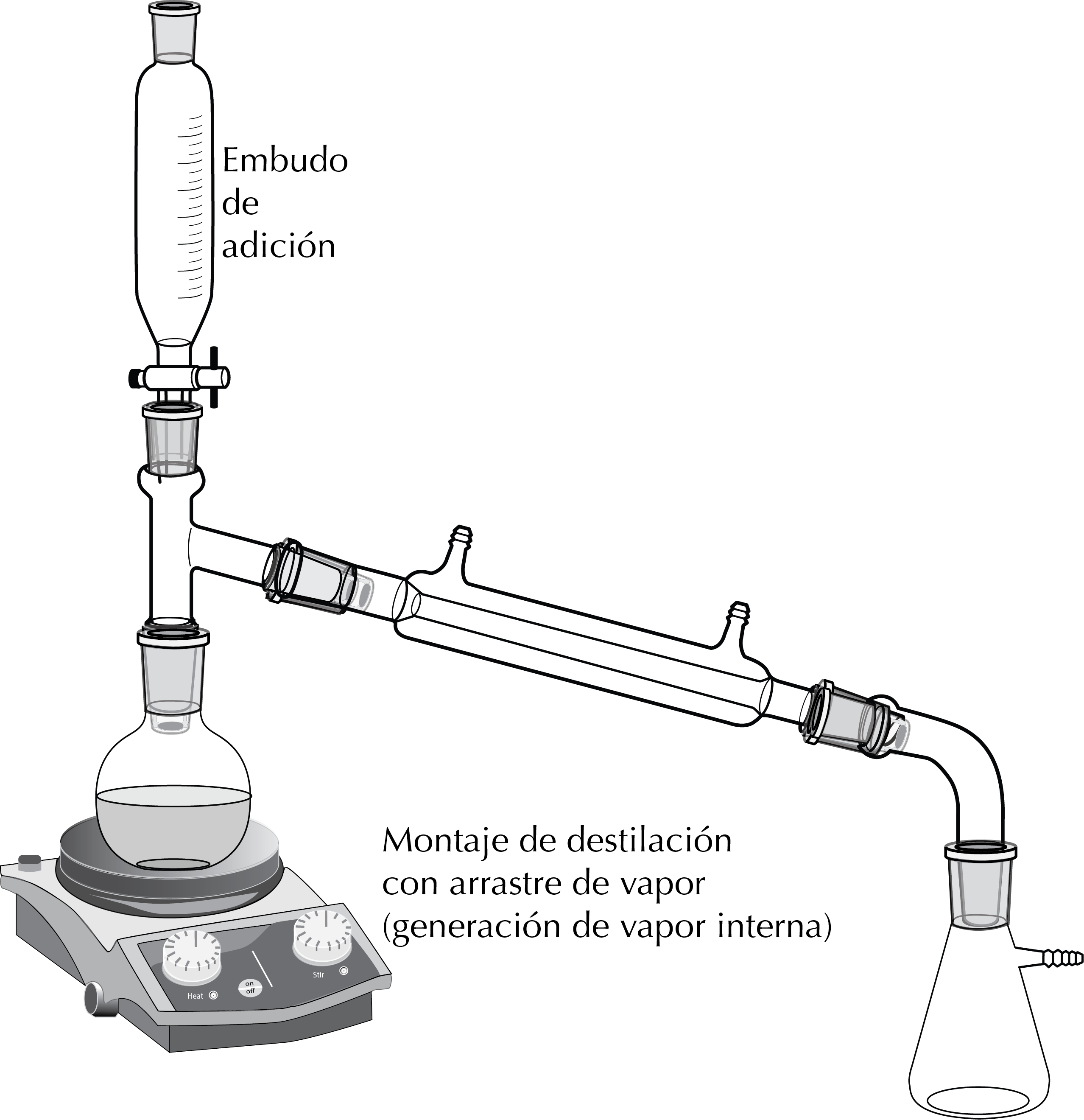
Distillation with internal steam source
In this case it is a distillation of a mixture of a product dissolved in an organic solvent and water. It is carried out as a simple distillation and an addition funnel can be placed in the set-up with water that is gradually added.
Vapor pressure of a mixture
Given a miscible mixture (homogeneous solution), the vapor pressure of each component depends on the vapor pressure of the pure component and its mole fraction in the liquid mixture according to Raoult’s law:
pTo = pTo* · xTo
where pA is the vapor pressure of a liquid component in a miscible liquid mixture, pA* is the vapor pressure of the pure liquid and xA is the mole fraction of that liquid in the mixture, which is equal to:
nTo / nt
Where nA is the number of moles of the individual liquid in the mixture and nt is the total number of moles of all liquids in the mixture.
The total vapor pressure above the miscible liquid mixture is equal to the sum of the partial vapor pressure of each component in it, which is known as Dalton’s law of partial pressures.
The vapor pressure of a liquid increases with temperature as more molecules gain kinetic energy to escape from the liquid phase to the gas phase. In a miscible mixture containing two liquids, the pressure is expressed as:
P = pTo + pB
Where pA and pB are the vapor pressures of liquid A liquid B, respectively, above the mixture. P is the total vapor pressure of both liquids above the mixture.
The combination of the equations describes the relationship between the total vapor pressure of the solution and the mole fraction of the individual components:
P = pTo* ·xTo + pB* ·xB
In an immiscible mixture, where the components form a heterogeneous mixture, the vapor pressures of each component contribute independently to the total vapor pressure.
Consequently, the total vapor pressure is equal to the sum of the individual pure vapor pressures.
In an immiscible mixture composed of two liquids, the total pressure is defined as the vapor pressure of the first liquid plus the vapor pressure of the second liquid.
P = pTo* + pB *
Boiling point of an immiscible mixture
As a liquid is heated, the vapor pressure increases. Each component of a mixture has its own boiling point. In a mixture of miscible liquids, boiling occurs at a temperature between the boiling points of the constituent liquids.
However, for an immiscible mixture, boiling occurs at a much lower temperature than the boiling points of the individual components. Since each individual component contributes independently, less heat is required to raise the total vapor pressure to atmospheric pressure.
For example, in the case of the immiscible mixture of benzene and water. The boiling points (b.p.) of benzene and water at 1 atm are 80.1 and 100 ºC, respectively. The solution boils when the total vapor pressure reaches 760 mm Hg (normal atmospheric pressure). At 69.3 ºC, the vapor pressure of water is 227 mm Hg and the vapor pressure of benzene is 533 mm Hg, which in total equals the 760 mm Hg required for boiling. Therefore, these values are well below the b.p. of each individual component.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2