Objetives
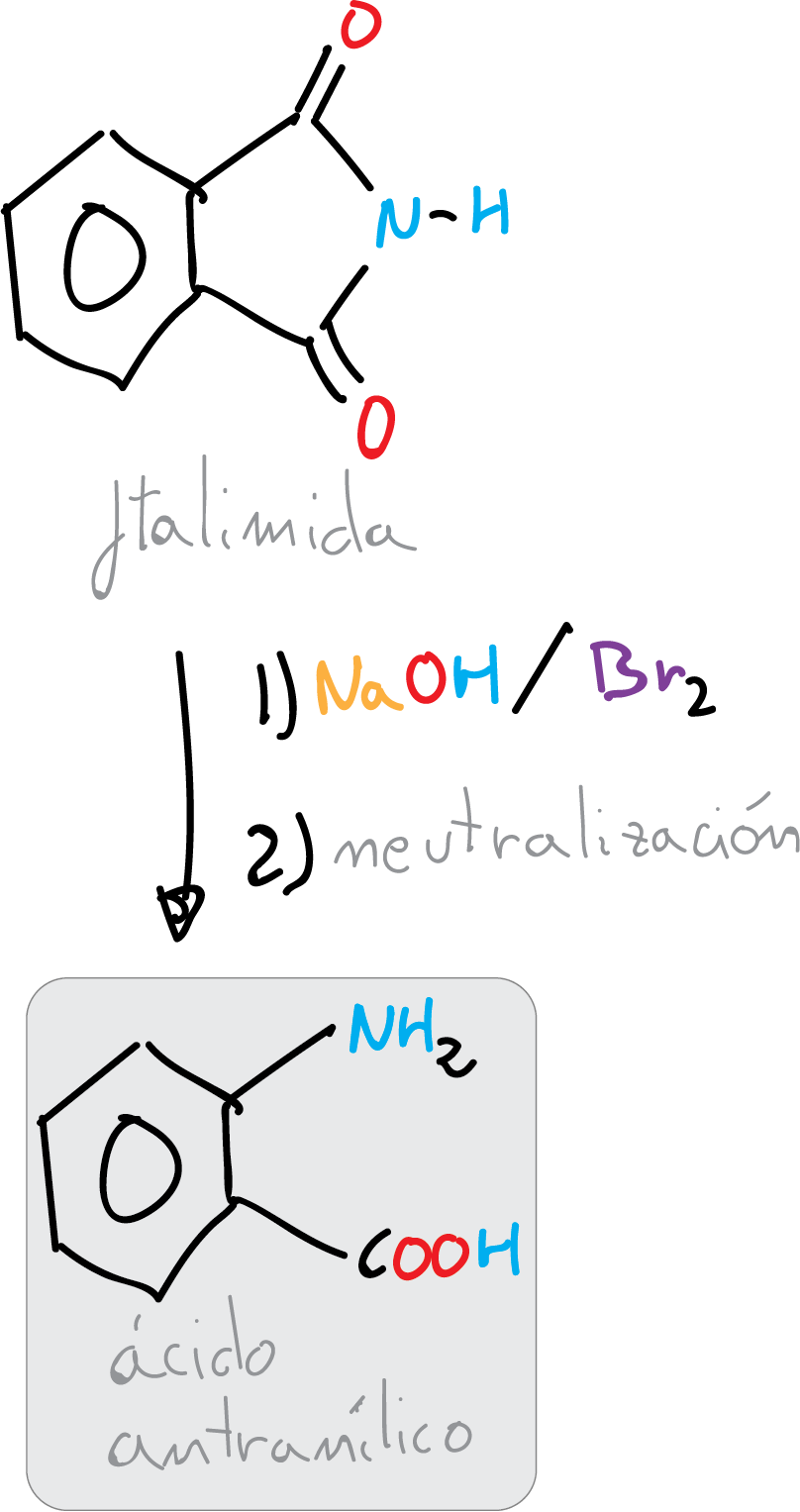
Synthesis of 2-aminobenzoic acid (anthranilic acid) from phthalimide by a bromine-promoted Hofmann transposition reaction in basic medium.
Background
The Hofmann transposition of an amide to form an amine with loss of carbon dioxide (CO2) is an example of a reaction in which alkyl or aryl groups migrate to electron-deficient nitrogen atoms.
Other examples of very similar reactions include the Curtius, Lossen and Schmidt rearrangements, in which a carboxylic acid or acid derivative is converted to an isocyanate group, producing the amine. In addition, a related process is the Beckmann transposition reaction, in which oximes are converted to amides.
In Hofmann transposition, an amide undergoes an oxidation process with hypobromite to form an N-bromoamide intermediate, which in the presence of a base undergoes a deprotonation step followed by migration of an alkyl group to the nitrogen atom, and simultaneous loss of bromine, thus generating an isocyanate.
Under the reaction conditions, hydrolysis of the isocyanate group occurs with the loss of CO2 and the formation of the corresponding amine, which has one carbon atom less than the starting amide. This reaction is especially useful for the preparation of aromatic amines.
In this experiment, the objective of this practice is to apply the Hofmann reaction to the synthesis of 2-aminobenzoic acid (anthranilic acid), which is used as a synthetic intermediate in the preparation of dyes or saccharin synthesis. Its esters are used in the preparation of perfumes, medicines or corrosion inhibitors in metals.
Experimental procedure
Dissolve 8 g of NaOH in 30 ml of deionized water in a 100 ml Erlenmeyer flask with magnetic stirring. Cool the solution in an ice bath and add at once 6.5 g of bromine (Br2). Stir the mixture vigorously until the brown color disappears, indicating that all the bromine has reacted. Then, while vigorously stirring, add 5.9 g of finely divided phthalimide and then add a solution of 5.5 g NaOH in 20 ml water. Remove the ice bath and allow the temperature to rise spontaneously to approximately 70 °C and maintain stirring for another 10 min.
If there is turbidity, filter by gravity. Cool the reaction crude again in an ice bath and add dropwise HCl (conc.) using a dropper to neutrality (will require about 15 ml), check the pH using indicator paper. If there is a slight excess of acid, adjust the pH by adding a base.
Transfer the reaction mixture to a 250 ml Erlenmeyer flask and add 5 ml of glacial acetic acid. Isolate the precipitate by vacuum filtration and wash with 10 ml of water. Recrystallize 2-aminobenzoic acid (anthranilic acid) in water and a yellowish solid will form (m.p. = 146-148 °C).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Anthranilic acid | 137.14 | 144-148 | - | - |
| Br2 | 159.81 | 7.2 | 58.8 | - |
| HCl | 36.46 | -30 | >100 | 1.200 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Phthalimide | 147.13 | 232-235 | 310 | 1.210 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic acid |   |
| Anthranilic acid |  |
| Br2 |    |
| HCl |   |
| NaOH |  |
| Phthalimide | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Anthranilic acid | RWZYAGGXGHYGMB-UHFFFAOYSA-N |
| Br2 | GDTBXPJZTBHREO-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Phthalimide | XKJCHHZQLQNZHY-UHFFFAOYSA-N |
Video on synthesis of p-aminobenzoic acid
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- C. M. Dougherty, R. L. Baumgarten, A. Sweeney, and E. Concepcion, Phthalimide, anthranilic acid, benzyne. An undergraduate organic laboratory sequence, Journal of Chemical Education 54 (1977), no. 10, 643, DOI: 10.1021/ed054p643