Objective
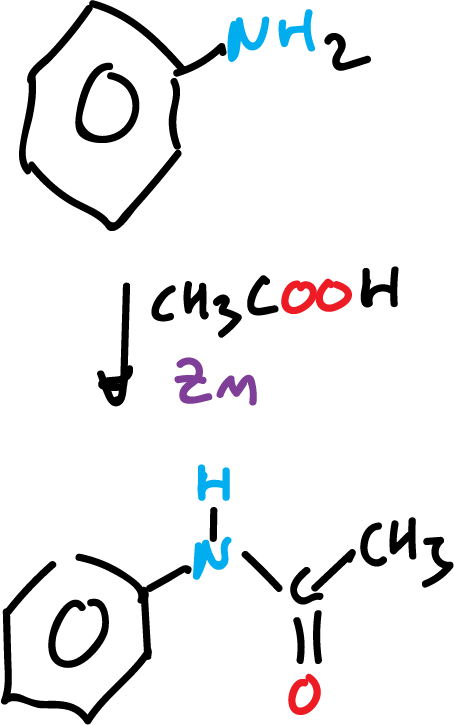
Alternative acetanilide synthesis route to obtain aromatic amides without using acetic anhydride. Using instead acetic acid as acylating agent catalyzed by zinc.
Background
The acetanilide synthesis, or acetamides in general, are usually carried out from an amine (aromatic or aliphatic) with acetic anhydride. Acetic anhydride, a reagent widely used in organic chemistry laboratories, irritates mucous membranes, is corrosive and has an unpleasant odor that requires work in a fume hood. In addition, it reacts violently with water. As an alternative to this procedure, the synthesis of an aromatic amide (acetanilide) using acetic acid as an acylating agent, catalyzed with zinc, is proposed.
Experimental procedure
In a 100 ml round bottom flask, prepare a mixture of 10 ml aniline, 0.5 g zinc powder and 30 ml acetic acid. Connect the flask to a reflux condenser and heat it in a water bath at 60 °C for 2 h with magnetic stirring.
After completion of the heating time, carefully pour the crude reaction mixture into a 250 ml Erlenmeyer flask containing 100 ml of cold water. Cool the Erlenmeyer in an ice water bath and stir the mixture with a glass rod. After a few minutes, acetanilide crystals will start to form. Remove them by filtration under vacuum and wash the solid with cold water until the acetic acid odor disappears. Recrystallize in water adding activated carbon if the product contains colored impurities.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetanilide | 135.16 | 113-115 | 304 | - |
| Aniline | 93.13 | -6 | 184 | 1.022 |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Acetic anhydride | 102.09 | -73.1 | 139.8 | 1.080 |
| Zinc (dust) | 65.39 | 420 | 907 | 7.133 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetanilide |  |
| Aniline |     |
| Acetic acid |   |
| Acetic anhydride |    |
| Zinc (dust) |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetanilide | FZERHIULMFGESH-UHFFFAOYSA-N |
| Aniline | PAYRUJLWNCNPSJ-UHFFFAOYSA-N |
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Acetic anhydride | WFDIJRYMOXRFFG-UHFFFAOYSA-N |
| Zinc (dust) | HCHKCACWOHOZIP-UHFFFAOYSA-N |
Video on acetanilide synthesis
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- V. K. Redasani, V. S. Kumawat, R. P. Kabra, P. Kansagara, and S. J. Surana, Applications of green chemistry in organic synthesis, International Journal of ChemTech Research 2 (2010), no. 3, 1856-1859