Objective
To familiarize the student with the preparation of Grignard reagents and their reaction with carbonyl compounds to form alcohols, in particular the synthesis of triphenylmethanol (an alcohol) from bromobenzene.
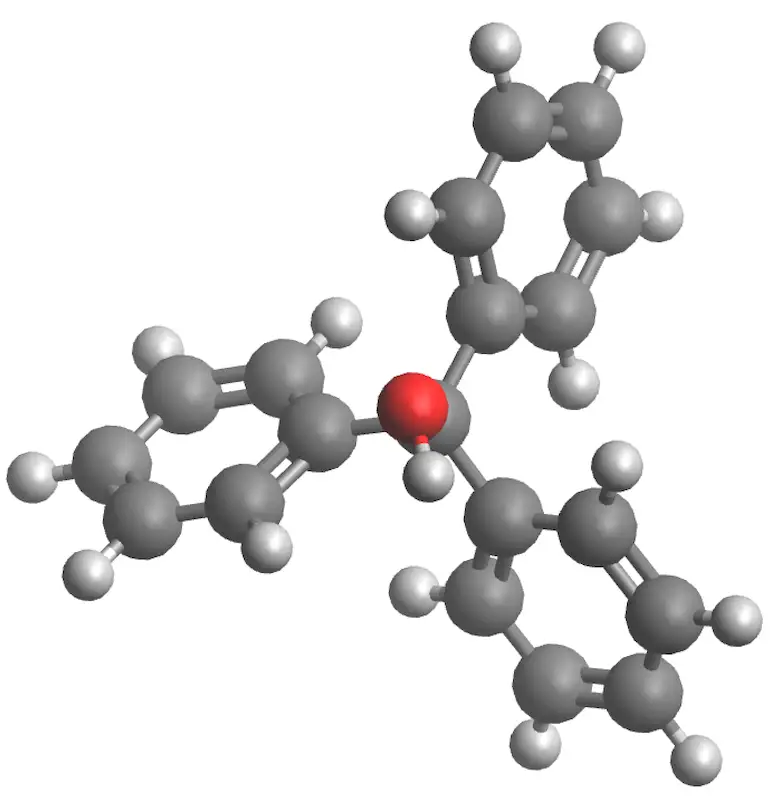 |
| 3D Structure |
Background
One of the most popular methods for forming C-C bonds in organic synthesis is by using Grignard reagents (Nobel Prize in Chemistry in 1912).
The formation of these involves the reaction of an alkyl, vinyl or aryl halide with magnesium and involves a change in the electronic nature of the carbon atom, which changes from electrophile in the halide to strongly nucleophile in organomagnesium compounds.
Grignard compounds are highly reactive, reacting with water, oxygen, CO2, etc. Therefore, these compounds must be prepared under anhydrous conditions using an inert atmosphere. They have a strongly nucleophilic (and basic) character and produce additions to the carbonyl groups, with the formation of a new C-C bond, giving rise to alcohols whose nature depends on the type of carbonyl starting compound used.
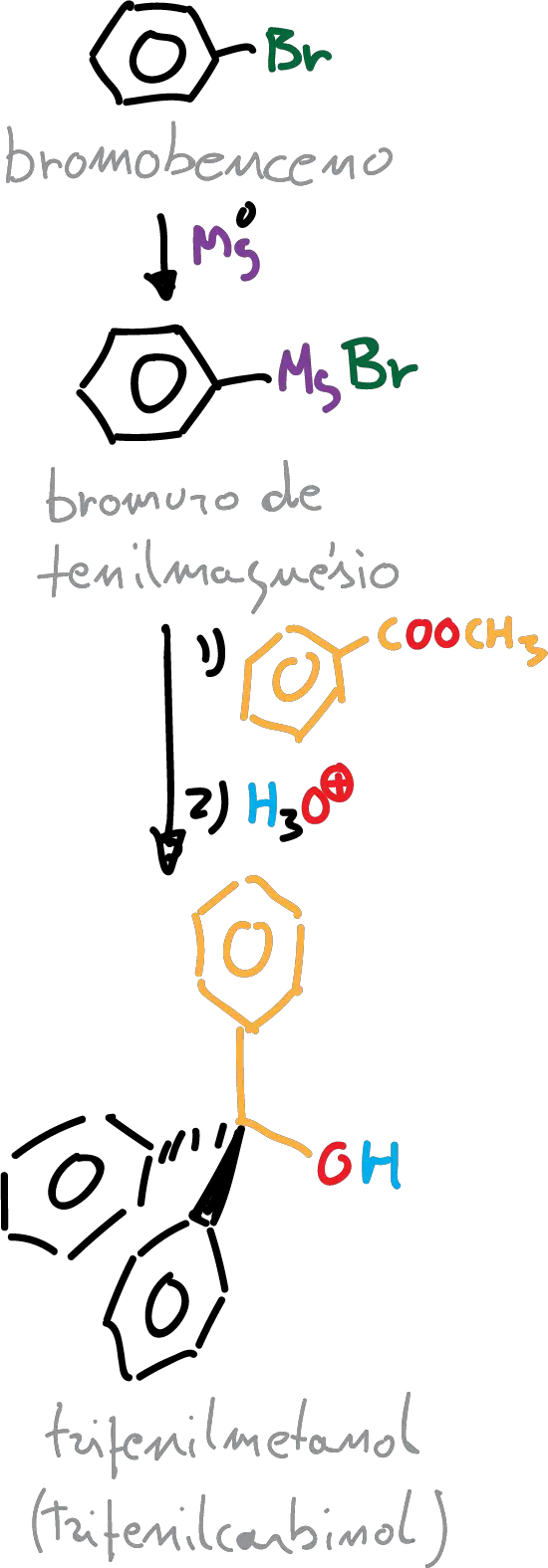
In this experiment, the reagent chosen is an ester (methylbenzoate), so the product formed is a tertiary alcohol, in this particular case triphenylmethanol (triphenylcarbinol).
Reaction mechanism
The reaction mechanism starts with a first stage where the Grignard reagent dissociates to form a carbanion (negative charge) which attacks the carbonyl carbon with positive partial charge.
Nucleophilic addition of Grignard reagent to methyl benzoate causes the methoxide to be the leaving group of the intermediate and benzophenone is formed.
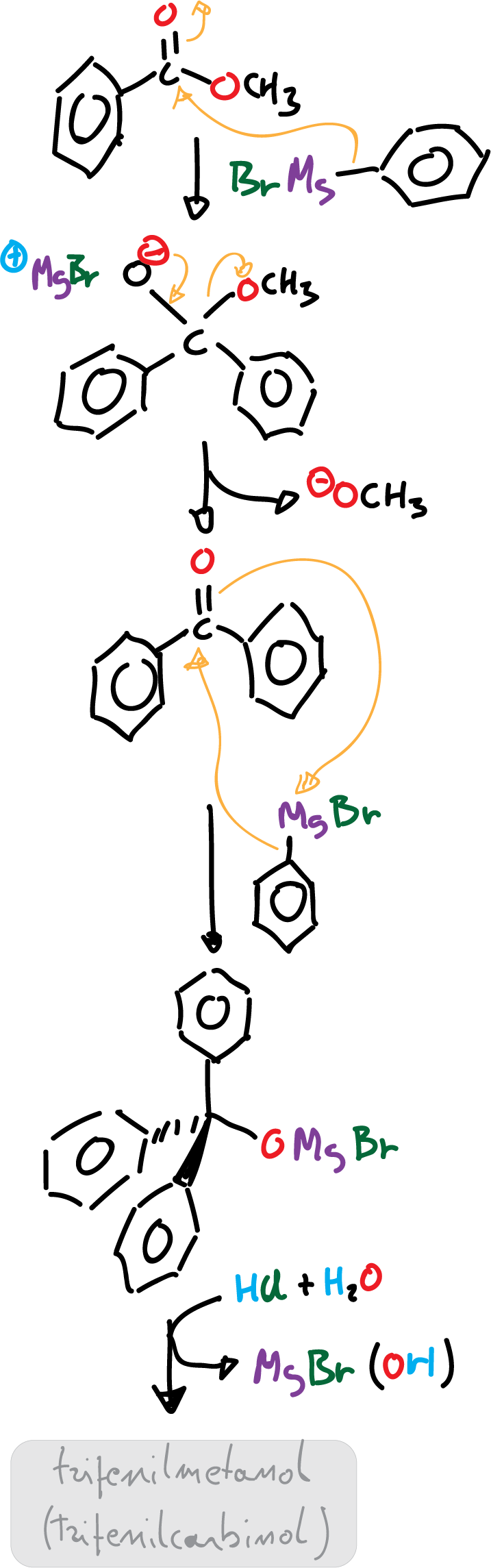
Since benzophenone also consists of a more reactive carbonyl carbon (because it is attached to two phenyl groups), it favors the second nucleophilic attack of Grignard reagent and the (Ph)3CO⊖ anion with three phenyl rings is produced. The acidic medium of the reaction protonates the (Ph)3CO⊖ anion to generate triphenylmethanol, (Ph)3C-OH as product.
Experimental procedure
A) Preparation of phenylmagnesium bromide:
Place 2 g of magnesium chips and 15 ml of anhydrous ether in a dry round bottom flask. The flask is fitted with the necessary elements for a reflux assembly under anhydrous conditions and with addition of reagents.
A solution of 9 g of bromobenzene in 10 ml of anhydrous ether is prepared and placed in the addition funnel. Then, an inert atmosphere is prepared by letting argon pass through the system for a few minutes and placing an argon-filled balloon over a septum. Add about 2 ml of bromobenzene solution over the magnesium and shake the reaction vigorously. After a few minutes, bubbles and a turbidity will appear (heating also occurs). Then, continue adding bromobenzene dropwise for 30 min with the addition funnel.
Once the addition is finished, wash the funnel with a few milliliters of ether. Keep the reflux for approximately 15-20 min more, heating gently if necessary.
A whitish reaction crude is formed, which should not contain magnesium. Allow to cool to room temperature, and use without isolate in the next step.
B) Triphenylmethanol synthesis
The same setup as in the previous step is used. Prepare a solution of 5 g of methylbenzoate in 15 ml of anhydrous ether and transfer this solution to the addition funnel. Start adding this solution dropwise to the organomagnesium compound.
When the addition is finished, reflux for another 30 min. Then, pour the reaction crude into a beaker with 50 ml of H2SO4(10 %) and about 25 g of ice. Carry over the remains of the flask with small portions of a cold H2SO4 solution. Stir the mixture, and when all the ice is melted, transfer it to a separatory funnel and decant (add a small amount of ether if there is a significant decrease in volume).
Re-extract the aqueous layer with ether (15 ml). Combine the ether extracts and dry over anhydrous sodium sulfate; then filter and decant into a flask with 25 ml hexane. The mixture is transferred to a rotary evaporator to produce a solid. Filter, wash with hexane, dry and weigh the solid.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Argon | 39.95 | -189.2 | -185.7 | - |
| Bromobenzene | 157.01 | -31 | 156 | - |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| Magnesium | 24.31 | 648 | 1,090 | 1.740 |
| Methyl benzoate | 136.15 | -12 | 198-199 | 1.094 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| Phenylmagnesium bromide | 181.31 | - | - | - |
| Triphenylcarbinol | 260.33 | 160-163 | 360 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Argon | Non-hazardous |
| Bromobenzene |    |
| Diethyl ether |   |
| H2SO4 |  |
| Hexane |     |
| Magnesium |  |
| Methyl benzoate |  |
| Na2SO4 | Non-hazardous |
| Phenylmagnesium bromide |    |
| Triphenylcarbinol |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Argon | XKRFYHLGVUSROY-UHFFFAOYSA-N |
| Bromobenzene | QARVLSVVCXYDNA-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| Magnesium | FYYHWMGAXLPEAU-UHFFFAOYSA-N |
| Methyl benzoate | QPJVMBTYPHYUOC-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| Phenylmagnesium bromide | NIXOIRLDFIPNLJ-UHFFFAOYSA-M |
| Triphenylcarbinol | LZTRCELOJRDYMQ-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- R. D. Pointer and M. A. G. Berg, Using a premade Grignard reagent to synthesize tertiary alcohols in a convenient investigative organic laboratory experiment, Journal of Chemical Education 84 (2007), no. 3, 483, DOI: 10.1021/ed084p483