Objetives
To perform a stereospecific synthesis, as well as to show the relevance of epoxides (oxacyclopropanes) as versatile intermediates in Organic Synthesis.
Background
In this experiment, trans-cyclohexane-1,2-diol is obtained from cyclohexene, prior to the transformation of the latter into trans-2-bromocyclohexanol by a stereoselective process (anti addition to a double bond), followed by the formation of an epoxide and subsequent opening of the latter.
Formally, the reaction results in the addition-anti of the hypobromous acid component (HOBr) to the double bond by reaction of cyclohexene with N-bromosuccinimide (NBS) in an aqueous medium. The intermediate (bromonium ion) initially formed reacts with water, which is the most nucleophilic species present (because almost no bromide ion concentration is possible).
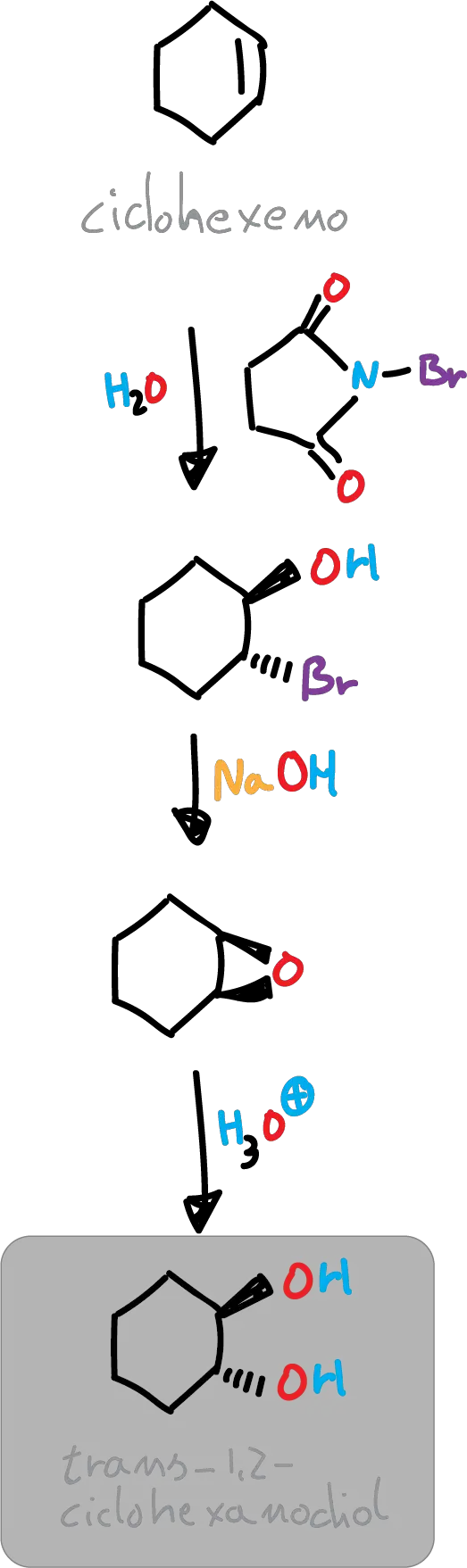
In this experiment, the bromohydrin intermediate is not isolated but is immediately converted into an epoxide. Treatment of trans-2-bromocyclohexanol with a base leads to the formation of the corresponding epoxide by an intramolecular nucleophilic substitution process. The available antiperiplanar arrangement of the OH and Br groups allows the formation of 1,2-epoxycyclohexane.
The opening of the epoxide takes place in acidic media, thus exerting steric control over the process, as the epoxide is protonated and induces the attack of water on the back face of the epoxide. The result is the formation of a trans diol.
Experimental procedure
A) Obtaining 2-bromocyclohexan-1-ol
To an Erlenmeyer flask with a magnet, add (in this order): 7.6 ml cyclohexene, 20 ml deionized water and 25 ml THF. Place the flask in an ice bath, over on a magnetically stirred plate and shake the reaction of 14.7 g NBS in 40 ml THF gradually for about 20 min. The reaction temperature should not exceed 30 °C. The temperature is monitored throughout the process by inserting a thermometer into the reaction carefully so that it is not struck by the stir bar. After the addition of NBS, keep stirring for a further 30 min. At the end of the 30 min reaction time, the reaction crude is poured into a separating funnel. Add 30 ml of diethyl ether and 30 ml of brine. The mixture is stirred and decanted. Separate the organic (upper) layer and extract the aqueous layer again with diethyl ether (2 x 20 ml). Combine the diethyl ether extracts and wash again with brine (2 x 20 ml) to prepare a solution of trans-2-bromocyclohexanol to be used in the next step.
B) Obtaining 1,2-epoxycyclohexane
To a 250 ml round bottom flask with fround-glass joints and Claisen adapter (with a magnet) or alternatively to a two-necked flask, add 25 ml of a 5M NaOH solution. Mount a reflux condenser and add slowly over 40 min, from the addition funnel, a solution of 2-bromocyclohexan-1-ol in ether, prepared in the previous step. While stirring, maintain the reaction temperature at approximately 40 °C. After addition, stir the mixture for 30 min. When addition is complete, transfer the mixture to an extraction funnel. Separate the two phases and dry the organic (upper) phase over sodium hydroxide granules, with occasional stirring for 15 min. Decant the solution and remove the solvent at the rotary evaporator. Distill the residue at atmospheric pressure (124-134 °C) and then weigh and calculate the yield of this step.
C) Obtaining trans-cyclohexane-1,2-diol
In a 100 ml round bottom flask, place 2 ml (1.95 g, 15 mmol) of 1,2-epoxycyclohexane. Adjust the quantities to the result obtained in the previous step. Add 10 ml of water and 1 ml of H2SO4 and shake the mixture vigorously for 1 h (stopper the flask). During this period of time, observe that the flask warms up and that the reaction mixture becomes transparent. After stirring for 1 h, adjust the pH of the solution to 7 by adding NaOH solution dropwise.
Extract the aqueous solution with ethyl acetate (3 x 15 ml). Dry the organic extracts over anhydrous sodium sulfate, remove the desiccant by gravity filtration and concentrate at rotovap to 1/3 volume. Then, introduce the ethyl acetate solution in an ice bath until a solid appears. Filter the solid under vacuum, dry with an air flow, weigh and calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 1,2-Epoxycyclohexane | 98.14 | - | 129-130 | 0.97 |
| 2-Bromocyclohexan-1-ol | 179.055 | 11.82 | 225.52 | 1.520 |
| Cyclohexene | 82.14 | -104 | 83 | 0.779 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Tetrahydrofuran | 72.11 | -108.0 | 65-67 | 0.89 |
| trans-Cyclohexane-1,2-diol | 116.16 | 101-104 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 1,2-Epoxycyclohexane |    |
| 2-Bromocyclohexan-1-ol | See MSDS |
| Cyclohexene |    |
| H2SO4 |  |
| NaOH |  |
| Tetrahydrofuran |   |
| trans-Cyclohexane-1,2-diol | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 1,2-Epoxycyclohexane | ZWAJLVLEBYIOTI-UHFFFAOYSA-N |
| 2-Bromocyclohexan-1-ol | AAMCLCZHZXKWRV-UHFFFAOYSA-N |
| Cyclohexene | HGCIXCUEYOPUTN-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Tetrahydrofuran | WYURNTSHIVDZCO-UHFFFAOYSA-N |
| trans-Cyclohexane-1,2-diol | PFURGBBHAOXLIO-PHDIDXHHSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Oxidation of Cyclohexene to trans-1,2-Cyclohexanediol Promoted by p-Toluenesulfonic Acid without Organic Solvents
Andreia A. Rosatella, Carlos A. M. Afonso, and Luís C. Branco
Journal of Chemical Education 2011 88 (7), 1002-1003
DOI: 10.1021/ed1006772