What are thiophenes?
Thiophenes are heterocyclic organic compounds, consisting of a 5-membered aromatic ring with four carbon atoms and a sulfur atom. Thiophene is a liquid with a boiling point of 84 °C, found in coal tar. It occurs as a contaminant of benzene, when obtained from coal tar.
fig-01
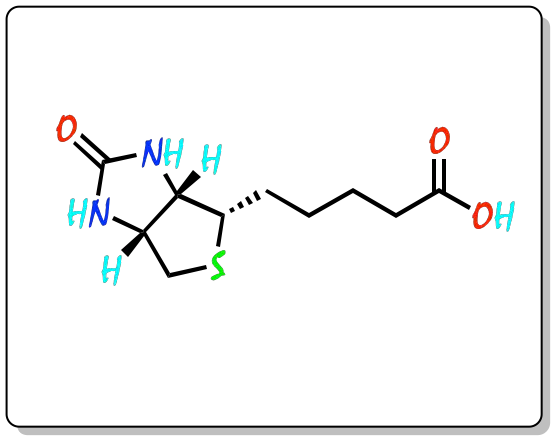
Thiophene is an electron-rich aromatic compound. Its resonance energy is approximately equal to that of pyrrole, and much higher than that of furan. It is found in nature in certain plant products, and is a component of many pharmaceuticals and synthetic dyes. Biotin (vitamin H) contains a tetrahydrothiophene ring in its structure and is found in yeast and eggs.
Synthesis of thiophenes
The commercial synthesis of thiophene is carried out by cyclization of butane, butadiene, or butenes with sulfur.
Thiophene can also be prepared on a laboratory scale by heating a mixture of sodium succinate and phosphorus trisulfide (P2S3), with yields of 25-30 %.
fig-02
Paal-Knorr synthesis of thiophenes
The Paal-Knorr synthesis of thiophenes is carried out from 1,4-dicarbonyl compounds with phosphorus pentasulfide(P2S5), in acidic medium.
fig-03
Hinsberg synthesis of thiophenes
Hisberg synthesis of thiophenes consists of the condensation of α-dicetones with ethyl thiodiacetate.
fig-04
The mechanism has been established by isotopic labeling experiments.
fig-05
Gewald synthesis of thiophenes
The Gewald synthesis of thiophenes consists of a cyclization of a compound I (α-carbonyl thiol) on nitriles.
fig-06
A simple carbonyl compound with an α-methylene group in the presence of elemental sulfur can be used instead of compound I (α-carbonyl thiol).
fig-07-nueva
The ring closure is reversible and some 2-amino thiophenes have been opened by reacting them with bases.
Electrophilic substitution reactions of thiophenes
Thiophene is somewhat less reactive than furan against electrophiles (which gives addition) and much less reactive than pyrrole (which gives substitution), but even so, it is much more reactive than benzene (103 times).
Because of its higher aromatic character, thiophene tends to undergo substitution rather than addition reactions, and it is not as easy to open the ring with acids as in the case of furan.
It is stable in aqueous mineral acids, but not in 100% sulfuric acid (H2SO4), nor in the case of strong Lewis acids such as aluminum chloride (Cl3Al).
Substitution is favored at the C2 position of the thiophene. Substitution at C3 has about a 1 % chance of occurring with most electrophiles.
Example of thiophene nitration
The nitration of thiophenes is carried out with nitric acid (HNO3) and acetic acid, and generates in a first step, the 2-nitro thiophene, and subsequently in another nitration step the major product 2,5-dinitro thiophene.
fig-08
In the case of 2-thiophene cyano nitration occurs at the C4 position.
fig-09
Example of sulfonation of thiophenes
Thiophene is sulfonated directly with sulfuric acid (H2SO4). In addition, this reaction is very practical because it is the usual procedure for releasing thiophene in benzene. It is carried out by stirring with sulfuric acid (H2SO4).
fig-09-nueva
Example of halogenation of thiophenes
This reaction is carried out with chlorine and bromine and results in the whole series of substitution products.
fig-10
Chlorination with chlorine and iodine produces the hexachlorine derivative.
fig-11
Effect of substituents on electrophilic substitution
The effect of the substituents on the thiophene ring can be summarized as follows
fig-12
On the other hand, the electrophilic attack on the sulfur atom of thiophenes is not a very common reaction.
The thiophene sulfur atom can be alkylated with a hard electrophile such as methyl fluorosulfonate (CH3SO3F).
fig-13
Peroxides and peroxyacids oxidize thiophene to give a sulfoxide, which is too unstable to be isolated.
fig-14
S,S-thiophene dioxide is an isolable but reactive diene.
fig-15
Neither of the above two compounds has an aromatic character.
Nucleophilic and radical substitution
Thiophenes substituted with electronattracting conjugating groups (especially the nitro group, –NO3) react with nucleophiles much more readily than in the case of benzene.
This is explained by the fact that the intermediate formed, Meisenheimer complex, has a carbanion adjacent to the sulfur that has a high capacity to stabilize it.
fig-16
These substitution reactions do not always follow the usual path. Thus, there are cases of cine-substitution, where the introduction of the nucleophile takes place on the atom adjacent to that of the leaving group.
fig-17
In addition, tele-substitution can also occur, when the introduction of the nucleophile takes place at an atom farther away than the cine-substitution.
On the other hand, nucleophiles, too, can cause ring opening.
fig-18
Thiophene can also be phenylated at the C2 position by reaction with aryl radicals, as shown in the following scheme.
fig-19
Thiophene can be readily transformed to 2-bromo thiophene with N-bromosuccinimide (NBS) by a mechanism believed to involve radicals.
fig-20
Under the conditions of this bromination reaction, of 2-methyl thiophene, no methyl halogenation occurs, but in the ring, giving compound I, 5-bromo-2-methyl thiophene, and only small amounts of the compound 2-bromomethyl thiophene.
Cycloaddition and addition reactions of thiophenes
Thiophene can give the Diels-Alder reaction with highly activated dienophiles, but much worse than in the case of furan.
It reacts only with maleic anhydride at 100 °C and high pressure. It also reacts with activated acetylenes and benzyne, but the adducts formed are unstable and lose the sulfur atom.
fig-21
Also, the [2+2] photochemical addition reaction of 2,5-dimethyl thiophene to ketones has been described.
Reduction reactions of thiophenes
It is difficult to reduce thiophenes using hydrogen and platinum catalyst (H2/Pt), because the noble metals in the catalyst are poisoned. However, with nickel Ni Raney the ring is opened and the sulfur is removed from the molecule.
fig-22
This reaction is used to synthesize saturated carbon compounds with functional groups in specific positions. For example, a large ring ketone as shown in the scheme.
fig-23
Thiophenes can be reduced with sodium in ammonia (Na/NH3) or methanol (Na/MeOH).
fig-24
Properties of some substituted thiophenes
Thiophene is more aromatic than furan and is not as strong an electron donor as pyrrole. Therefore, the substituents on the thiophene ring more closely resemble their benzene analogs.
Thus, thiophene-2-carboxaldehyde resembles benzaldehyde in almost all its chemistry and thiophene-2-carboxylic acid resembles benzoic acid, although it is slightly stronger.
fig-25
- 2-chloromethyl thiophene forms a Grignard derivative and undergoes nucleophilic shift reactions.
- The simple hydroxy and amino derivatives are highly reactive and easily oxidized as in the furan and pyrrole series.
- The 2-hydroxy derivatives exist as keto tautomers. However, the simple 3-hydroxy thiophenes are in the enolic form.
- If the thiophene ring has an adjacent electron-attracting group, then they become more stable. For example, aminocompounds can be diazotonated and copulated with the diazonium salt, as is the case with benzene aromatic amines (e.g. see synthesis of methyl orange).